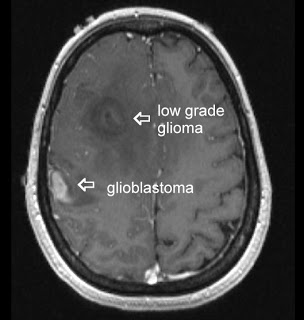
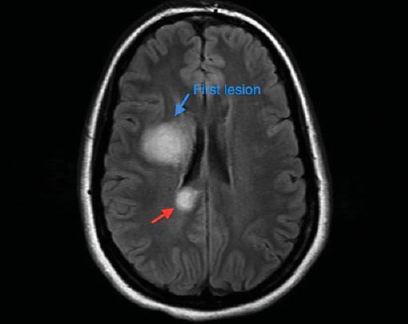
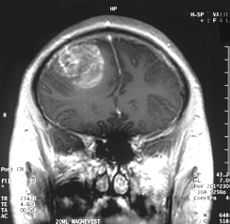
Glioblastoma multiforme (GBM) is the most common and lethal type of malignant primary brain tumors that account for over 70% of all intracranial cancers. The current course of GBM treatment consists of surgical resection of the main tumor mass, followed by administration of radiation and chemotherapy. Surgical resection of the primary tumor leads to injury to the surrounding normal tissue, while chemotherapy and radiotherapy cause toxicity to the healthy tissue in the brain. These undesirable secondary effects of glioma treatments have a major impact on patients’ physical, cognitive and emotional functioning. Nonetheless, despite this aggressive treatment regimen and its harmful side effects, GBM remains virtually incurable, with post-diagnosis median survival persisting less than 14 months. This dismal prognosis is due to a combination of unique anatomical features of the central nervous system (CNS), in addition to GBMs’ (glioma cells’) exceptional invasive capacity. Glioma cells infiltrate the brain’s highly dense parenchyma, migrating along the corpus callosum and creating new masses within the hemisphere contralateral to the initial tumor mass homing.
type of malignant primary brain tumors that account for over 70% of all intracranial cancers. The current course of GBM treatment consists of surgical resection of the main tumor mass, followed by administration of radiation and chemotherapy. Surgical resection of the primary tumor leads to injury to the surrounding normal tissue, while chemotherapy and radiotherapy cause toxicity to the healthy tissue in the brain. These undesirable secondary effects of glioma treatments have a major impact on patients’ physical, cognitive and emotional functioning. Nonetheless, despite this aggressive treatment regimen and its harmful side effects, GBM remains virtually incurable, with post-diagnosis median survival persisting less than 14 months. This dismal prognosis is due to a combination of unique anatomical features of the central nervous system (CNS), in addition to GBMs’ (glioma cells’) exceptional invasive capacity. Glioma cells infiltrate the brain’s highly dense parenchyma, migrating along the corpus callosum and creating new masses within the hemisphere contralateral to the initial tumor mass homing.
Thus, recurrence in postoperative GBM patients is in essence inevitable. Furthermore, GBMs are not only heterogeneous among individual patients, but they are also highly heterogeneous within a single tumor mass. Recent studies have shown compelling evidence of a therapeutically resistant subpopulation of malignant glioma cells that exhibit stem-cell like characteristics, such as multipotency, the ability to self-renew and invade/migrate; these tumor-initiating cells are referred to as glioma stem cells (GSCs) and are believed to be responsible for tumor initiation and recurrence in GBM patients. Furthermore, GSCs have been observed to ensconce within similar niches to neural stem cells (NSCs).
NSCs and mesenchymal stem cells (MSCs) have been shown to have an exceptional migratory ability within brain’s parenchyma and possess a notable inherent tumor tropism. Thus, several cell-based therapy (CBT) studies and clinical models of malignant tumors have incorporated autonomous tracking of tumor cells by employing NSCs and MSCs to deliver multiple therapeutic genes to specific tumor loci. This target specific drug-delivery model has NSCs or MSCs transduced to express one pro-drug-activating enzyme, which catalyzes the conversion of a particular pro-drug into an active toxic agent, which results in the localization of the chemotherapeutics specifically at the tumor sites. In addition to its ability to deliver effective cytotoxic damage to the tumor without causing damage to the healthy surrounding tissue, the resulting bystander effect of this system causes cell death not only to the drug-delivery-vehicle cells, but also to the surrounding glioma cells. Although a number of different enzyme/prodrug systems have been utilized in relevant studies, HSV-thymidine kinase (HSV-tk)/ deoxyguanosine analog ganciclovir (GCV) has been the most commonly tested in animal and in vitro models. HSV-tk phosphorylates GCV and produces deoxyguanosine triphosphate which is a polymerase-I inhibitor and DNA chain terminator; cell death occurs upon incorporation of the nucleotide analog within DNA chains.
In recent study published in the journal of Molecular Therapy, Blanco’s group report their findings regarding the interaction between human MSCs (hMSCs) and gliomas and the underlying mechanism for the effectiveness of hAMSC based therapy in GBMs. In their previous work Blanco’s group showed that the administration of hAMSCs expressing HSV thymidine kinase in glioma tumors significantly promotes tumor growth, whereas induction of cytotoxicity by administration of the prodrug GCV demonstrated a significant antitumor response.
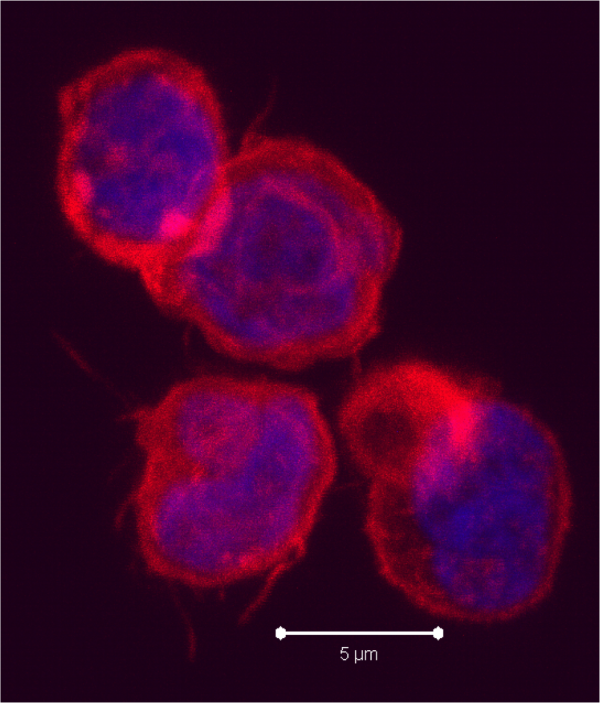
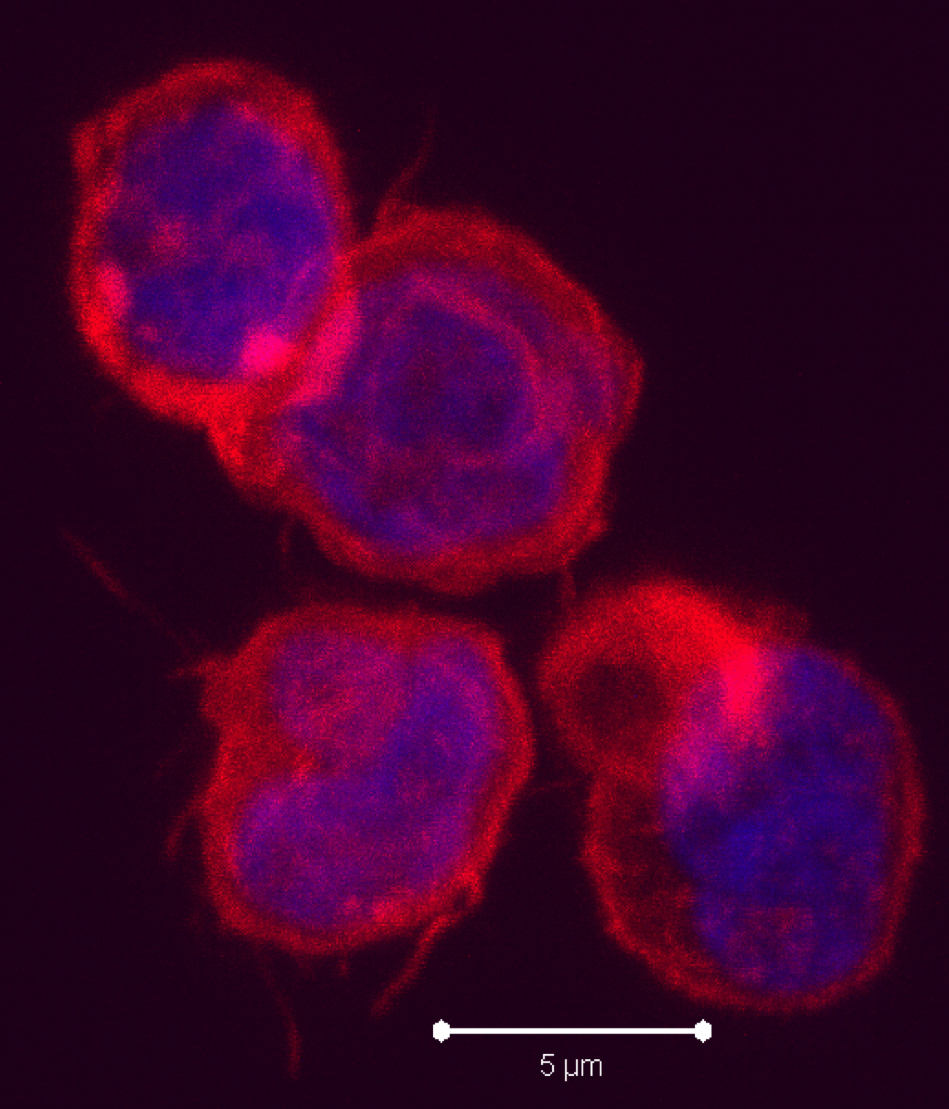
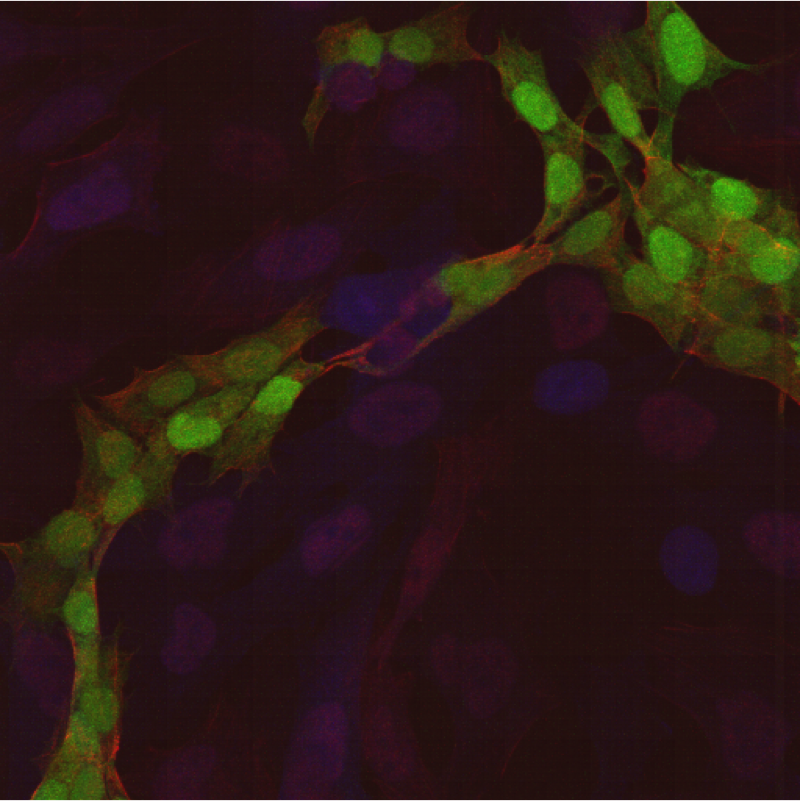
According to this new study, hMSCs differentiate to endothelial lineage (supported by the expression of CD31 marker) within tumors, and integrate in the tumor vascular system where they adopt an endothelial phenotype. Further, Blanco proposed the notion of hMSCs’ ability to home to privileged vascular structures where GSCs also reside, is the underlying characteristic that leads to the effectiveness of cytotoxic hMSCs in regulating the bystander killing of tumor cells.
Although Blanco’s study provides invaluable insights into the GSCs’ niche and its role in malignant brain tumor CBT, hMSCs’ tumor growth promotion is a quality, which makes these cells less than ideal for utilization in human clinical trials in the near future. Future studies on this mechanism using primary patient tumor cells rather than the U87 glioma cell line and human NSCs are needed to further confirm these observations.
Further reading:
Juli R. Bagó, Maria Alieva, Carolina Soler, Núria Rubio, Jerónimo Blanco. Endothelial differentiation of adipose tissue-derived mesenchymal stromal cells in glioma tumors: implications for cell based therapy. Molecular Therapy.

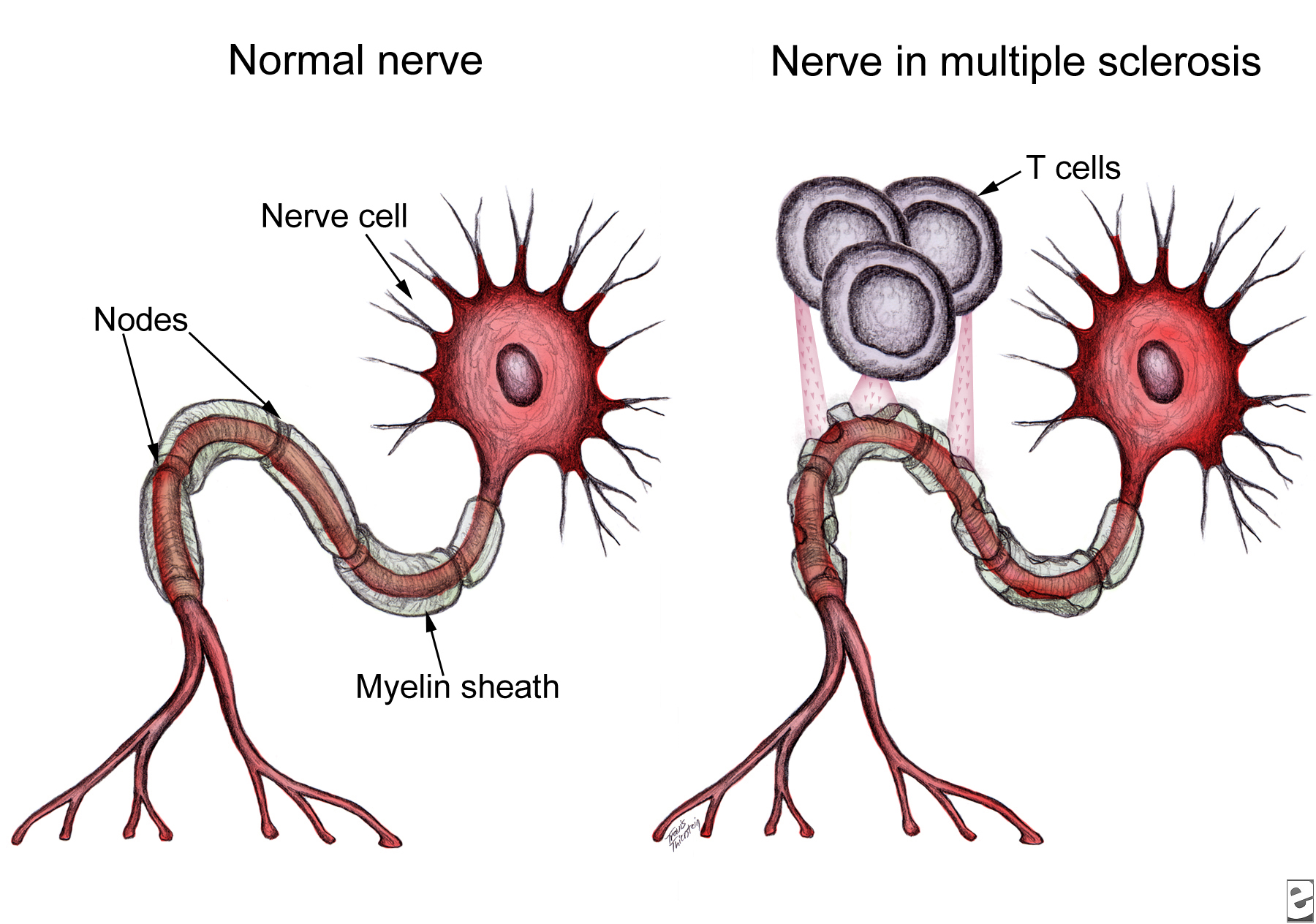

 These results support the epitope-spreading hypothesis, which indicates that MS patients make antibodies against one or a few myelin proteins, but as the disease progresses, the autoimmune response spreads to other myelin sheath epitopes. In their recent publication, Lutterotti et al. provide sufficient evidence necessitating emphasis not only on the specific target antigens, but also on the facility to inhibit epitope spreading, preferably prior to diversification of the
These results support the epitope-spreading hypothesis, which indicates that MS patients make antibodies against one or a few myelin proteins, but as the disease progresses, the autoimmune response spreads to other myelin sheath epitopes. In their recent publication, Lutterotti et al. provide sufficient evidence necessitating emphasis not only on the specific target antigens, but also on the facility to inhibit epitope spreading, preferably prior to diversification of the