Innate lymphoid cells (ILCs) are subsets of lymphoid cells that do not rearrange their antigen receptors like T cells and B cells but have other features of lymphocytes. ILCs include the Natural Killer (NK) cell subset, as well as cells that behave similarly to T helper cell subsets by producing similar characteristic cytokines. Type 2 ILCs (ILC2) resemble TH2  cells in that they produce IL-5 and IL-13. RORγt+ ILCs aka ILC3s, include subsets that resemble TH17 and TH22 cells by producing IL-17 and IL-22, respectively, as well as a subset that produces both cytokines. Several recent articles have identified another class of ILCs in both humans and mice. These cells resemble TH1 cells in that they express T-bet/TBX21 and produce IFN-gamma, and are distinct from conventional NK cells found among peripheral blood mononuclear cells (PBMC). These newly characterized cellular subsets have been denoted as Type 1 ILCs (ILC1).
cells in that they produce IL-5 and IL-13. RORγt+ ILCs aka ILC3s, include subsets that resemble TH17 and TH22 cells by producing IL-17 and IL-22, respectively, as well as a subset that produces both cytokines. Several recent articles have identified another class of ILCs in both humans and mice. These cells resemble TH1 cells in that they express T-bet/TBX21 and produce IFN-gamma, and are distinct from conventional NK cells found among peripheral blood mononuclear cells (PBMC). These newly characterized cellular subsets have been denoted as Type 1 ILCs (ILC1).
In the April 2013 issue of Immunity, Fuchs et. al sought to more fully characterize the ILC subsets present in human mucosal lymphoid tissues. In human tonsils, a CD3–CD56+ NKp44+CD103+ subset was identified that expressed T-bet and produced IFN-gamma when stimulated with either PMA/ionomycin, IL-12, or IL-15. These cells also expressed perforin and granzyme and had cytolytic activity. Although these cells express CD56+and NKp44+, which are markers characteristic of NK cells, they appear to be related to but distinct from prototypical CD56hi NK cells found in PBMC. For instance, unlike CD56hi PBMC NK cells, these ILC1 did not exhibit a response to IL-18, as measured by a synergistic production of IFN-gamma when stimulated with IL-12+ IL-18 vs. IL-12 alone.
In a separate study, published in the March 2013 issue of Nature Immunology, Bernink et. al identified a mucosal human ILC1 subset in tonsils that differs from that found by Fuchs et. al, being CD56–NKp44− as well asCD127+ and c-Kit−. Similarly to the cells described by Fuchs et. al, these cells expressed T-bet and produced IFN-gamma when stimulated with PMA/ionomycin or IL-12. However, they did not express perforin and granzyme. Additional characterizations differentiated these cells from NK cells including the lack of the KIR3DL1 and IL-15Rα markers expressed by NK cells.
ILCs have been found to reside in mucosal associated lymphoid tissues include the oral, lung, and gastrointestinal mucosa, and are thought to function in immune responses to pathogens as well as in tissue repair. ILCs including ILC3s have also been found to participate in inflammatory disease pathogenesis. Both types of ILC1 cells were shown to be increased in the intestinal mucosa of Crohn’s disease patients, although their exact locations differed. CD56+ NKp44+CD103+ cells were found to accumulate in the intraepithelial layer while CD127+CD56–c-Kit−NKp44− cells were found in the lamina propria. Thus, these two subsets of ILC1 cells differ in multiple aspects including tissue localization.
In conclusion, both types of ILC1 cells identified in these studies are distinct from conventional PBMC CD56hi NK cells, express T-bet, and produce IFN-gamma in response to IL-12 and IL-15 stimulation. Notably, ILC3 cells also heterogeneously express CD56, IFN-gamma, granzymes and perforin. Thus, many questions remain as to the functional and developmental differences between different ILC subsets and between CD56+ ILC1 cells and PBMC NK cells that reside in various tissues.
Reading:
Intraepithelial Type 1 Innate Lymphoid Cells Are a Unique Subset of IL-12- and IL-15-Responsive IFN-γ-Producing Cells. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Immunity. 2013 Apr 18;38(4):769-81.
Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjösberg JM, Spits H. Nat Immunol. 2013 Mar;14(3):221-9.
ILC1 Populations Join the Border Patrol. Maloy KJ, Uhlig HH. Immunity. 2013 Apr 18;38(4):630-2. doi: 10.1016/j.immuni.2013.03.005.
Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Spits H, Cupedo T. Annu Rev Immunol. 2012;30:647-75.
A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. Nature. 2013 Feb 14;494(7436):261-5.

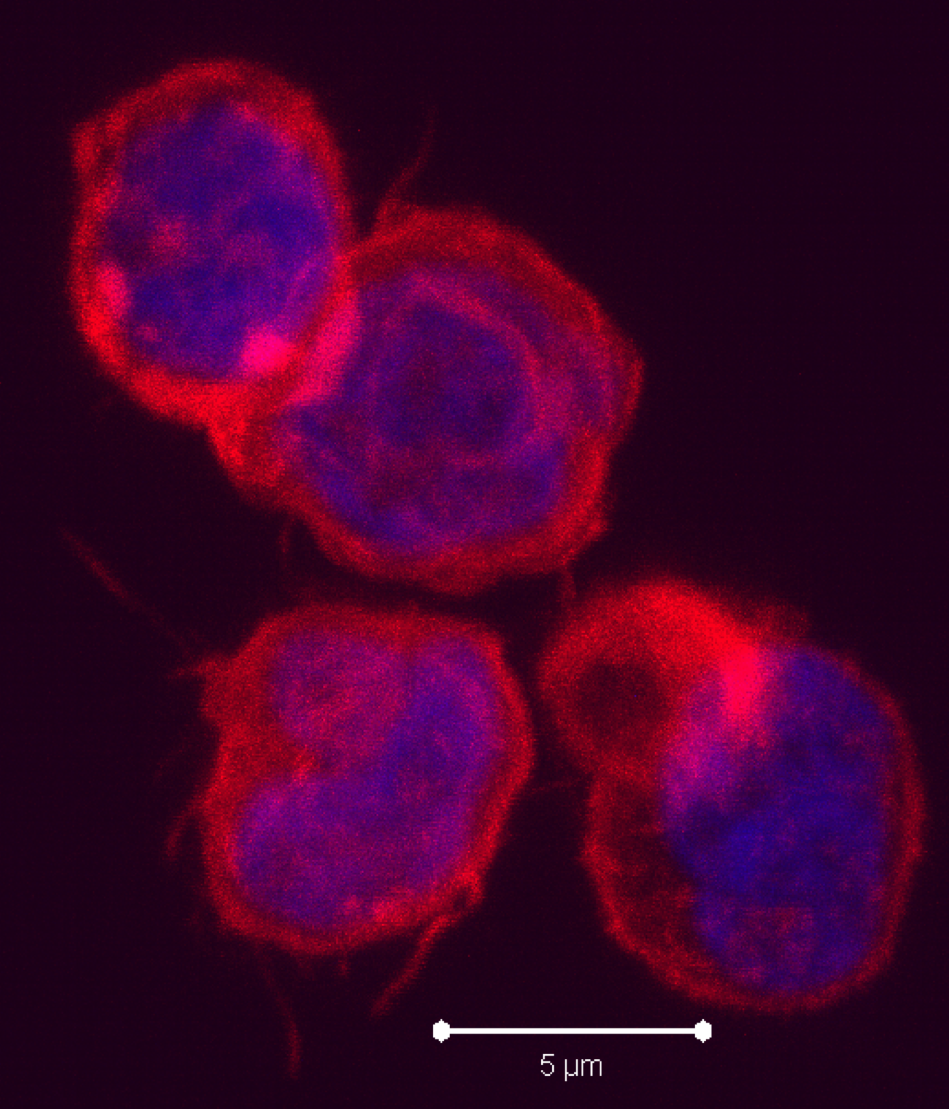

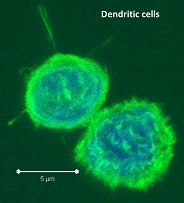
 -presentation is a DC-specialized mechanism by which antigens are taken up through endocytic and phagocytic pathways but presented in the context of MHC-class I, to activate antigen-specific cytotoxic CD8 T cells. Recent studies have sought to characterize the differences between the many tissue-associated DC subsets including their ability to cross-present antigen.
-presentation is a DC-specialized mechanism by which antigens are taken up through endocytic and phagocytic pathways but presented in the context of MHC-class I, to activate antigen-specific cytotoxic CD8 T cells. Recent studies have sought to characterize the differences between the many tissue-associated DC subsets including their ability to cross-present antigen.


 Negative regulatory CD4 T cells are well characterized and highly studied. However their CD8 counterparts are not well defined, particularly in humans. Regulatory CD8 T cells suppress activated CD4 T cells and have proposed roles in various human diseases including multiple sclerosis, ovarian carcinoma and infection with HIV, and many subsets have been described using various markers. In a recent issue of PLoS One, Hu et. al, describe a population of CD3+CD8+CD161−CD56+ T cells within human peripheral blood mononuclear cells (PBMC) that exhibit a cytolytic negative regulatory function.
Negative regulatory CD4 T cells are well characterized and highly studied. However their CD8 counterparts are not well defined, particularly in humans. Regulatory CD8 T cells suppress activated CD4 T cells and have proposed roles in various human diseases including multiple sclerosis, ovarian carcinoma and infection with HIV, and many subsets have been described using various markers. In a recent issue of PLoS One, Hu et. al, describe a population of CD3+CD8+CD161−CD56+ T cells within human peripheral blood mononuclear cells (PBMC) that exhibit a cytolytic negative regulatory function.
 Flow cytometry has been around since the 1950s when Wallace Coulter developed the first flow cytometry device and fluorescence-based flow cytometry was introduced in 1968 by Wolfgang Göhde. Since then, fluorescence-based flow cytometry and fluorescence-activated cell sorting (FACS) have blown up to become a mainstay of analytical scientific approaches in every field of cell biology, especially immunology. However, the dominance of fluorescence-based flow cytometry for analytical cellular biology may change with the recent introduction of a new technology: Time of Flight Mass Cytometry (CyTOF).
Flow cytometry has been around since the 1950s when Wallace Coulter developed the first flow cytometry device and fluorescence-based flow cytometry was introduced in 1968 by Wolfgang Göhde. Since then, fluorescence-based flow cytometry and fluorescence-activated cell sorting (FACS) have blown up to become a mainstay of analytical scientific approaches in every field of cell biology, especially immunology. However, the dominance of fluorescence-based flow cytometry for analytical cellular biology may change with the recent introduction of a new technology: Time of Flight Mass Cytometry (CyTOF).

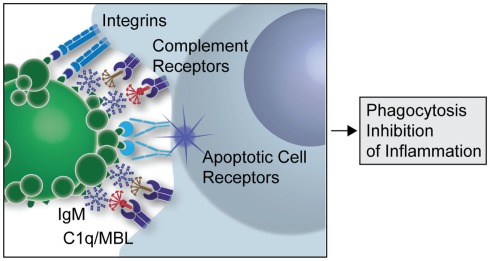
 Figure 1: A simplified overview of the clearance of apoptotic cells by natural IgMs via recruitment of complement factors (Silverman 2012)
Figure 1: A simplified overview of the clearance of apoptotic cells by natural IgMs via recruitment of complement factors (Silverman 2012)
 Arijit
Arijit
