The field of regenerative medicine holds great promise as we gain greater understanding of how stem cells differentiate into the many cell types found in our bodies. However, the clinical applications of these stem cells have been hampered by the challenges in replicating the in vitro capabilities of these cells once transplanted into the body. This is partially due to the fact that most stem cell work is based on culturing cells in a flat two-dimensional (2D) format.
Cell culture in 2D has been routinely used in laboratories for over 40 years. Cells are grown on flat dishes made of coated or uncoated polystyrene plastic or glass that are very stiff and arguably primitive. These cells attach and spread on the surface to form unnatural cell attachments to other cells and to deposited proteins that are denatured on this synthetic surface. Thus, the culturing of cells in 2D does not accurately reproduce the extracellular matrix (ECM) found in native tissue resulting in an alteration of many complex biological responses. The native microenvironment provides mechanical signals, soluble factors, communication between neighboring cells and communication between the cell and its matrix. This spatial and temporal organization affects normal cell fate including division, proliferation, migration, differentiation and apoptosis.
To overcome these challenges scientists have developed several three-dimensional (3D) culture methods such as cell spheroids, micro-carrier cultures, scaffolds, or tissue-engineered models. Cell spheroids, self-assembled spherical clusters of cell colonies, are simple, reproducible and similar to physiological tissues compared to other methods involving ECM scaffolds and hydrogel systems (water-swollen polymer networks). They are created from single culture or co-culture techniques such as hanging drop, rotating culture, non-adhesive surfaces or concave plate methods. A similar method is the development of epithelial tissues to form polarized sheets. However, as the size and complexity of the 3D model increases, so does the requirement for a scaffold which will ideally produce features naturally found within the ECM required for native cell function.
In this 3D cell culture environment, cells synthesize and secrete a flexible and pliable extracellular matrix in their native configuration. Gap junctions are increased in 3D culture allowing cells to communicate with each other via exchange of ions, small molecules and electrical currents. Surface adhesion molecules and receptors critical for cell function are also maximized. The various effects of 3D culture versus 2D culture on differentiation, drug metabolism, expression, cell function, morphology, proliferation, viability, response to stimuli and in vivo relevance are vast and ever expanding (JUST THE FACTS: Specific effects of 3D vs. 2D cell culture).
In a recent study published April 11, 2013 online in Advanced Functional Materials, researchers at Case Western University describe how micropatterning technology influences stem cell fate decisions. Micropatterning technology is the use of a technique to influence the network pattern of microgels aka intelligent hydrogel systems. While this technology has recently become an important tool for spatially controlling stem cell microenvironment, very little is known about the effect of the size of the micropatterned regions, which influences hydrogel stiffness and transport properties, on stem cell behavior. The researchers developed a 3D micropatterned hydrogel system that was either single-crosslinked or dual-crosslinked and evaluated human adipose-derived stem cell (hASC) behavior. The cells grew into clusters in the singly-crosslinked regions where the size of the hASC clusters depended on the micropattern size (increased cell-cell interactions may have promoted cell proliferation), while hASCs encapsulated in the dual-crosslinked regions remained mostly isolated and had lower proliferation rates. Interestingly, osteogenic (bone) and chondrogenic (cartilage) differentiation of the hASCs increased as the micropattern size increased but there was no effect on adipogenic (fat cells) differentiation. The researchers believe that controlling local biomaterial properties may allow them to guide the formation of complex tissues.
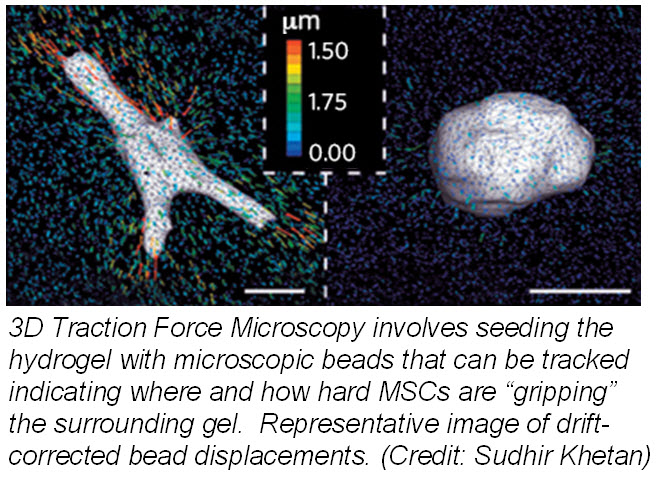
Another study published March 24, 2013 in Nature Materials describes how mechanotransduction (how cells take information about its physical environment and t ranslate that into chemical signals) can influence stem cell fate. Researchers from University of Pennsylvania showed that cell fate is regulated by cell-generated tension that is enabled through cell-mediated degradation of the covalently crosslinked matrix. When cultured on “softer” 2D covalently crosslinked gels (RGD-modified methacrylated hyaluronic acid hydrogels), mesenchymal stem cells (MSCs) differentiated into adipocytes when cultured in bipotential adipogenic/ osteogenic media. In contrast, MSCs cultured on “harder” 2D alginate gels differentiated into chondrocytes. This phenomenon was not present in 3D hydrogels and was attributed to the inability of cells to degrade the covalent cross-linked bonds resulting in MSCs differentiating into adipocytes. Introduction of proteolytically cleavable crosslinks and utilizing 3D traction force microscopy, revealed that MSC differentiation into bone cells was dependent on the cells to better anchor themselves into the environment and degradation signals.
ranslate that into chemical signals) can influence stem cell fate. Researchers from University of Pennsylvania showed that cell fate is regulated by cell-generated tension that is enabled through cell-mediated degradation of the covalently crosslinked matrix. When cultured on “softer” 2D covalently crosslinked gels (RGD-modified methacrylated hyaluronic acid hydrogels), mesenchymal stem cells (MSCs) differentiated into adipocytes when cultured in bipotential adipogenic/ osteogenic media. In contrast, MSCs cultured on “harder” 2D alginate gels differentiated into chondrocytes. This phenomenon was not present in 3D hydrogels and was attributed to the inability of cells to degrade the covalent cross-linked bonds resulting in MSCs differentiating into adipocytes. Introduction of proteolytically cleavable crosslinks and utilizing 3D traction force microscopy, revealed that MSC differentiation into bone cells was dependent on the cells to better anchor themselves into the environment and degradation signals.
These two studies provide insight into how the microenvironment can affect the fate of stem cells. Understanding how the microenvironment influences stem cell behavior is important for tissue engineering approaches. Cell-based assays have the potential to provide reliable data for regenerative medicine but scientists need to bridge the in vitro and in vivo gap by growing cells within a microenvironment that establishes physiological cell-cell and cell-substrate interactions that regulate proliferation and differentiation. Hence, 3D models will provide more reliable and meaningful therapeutic results compared to 2D tests.
Further reading:
Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011; 695:1-15
Oju Jeon, Eben Alsberg. Regulation of Stem Cell Fate in a Three-Dimensional Micropatterned Dual-Crosslinked Hydrogel System. Advanced Functional Materials. Article first published online: 11 April 2013
Wei Song, Naoki Kawazoe, and Guoping Chen. Dependence of Spreading and Differentiation of Mesenchymal Stem Cells on Micropatterned Surface Area. Journal of Nanomaterials. Volume 2011 (2011), 9 pages
Sudhir Khetan, Murat Guvendiren, Wesley R. Legant, Daniel M. Cohen, Christopher S. Chen and Jason A. Burdick. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials. Published online 24 March 2013