Inhibition of cell death or apoptosis has been implicated in the chemotherapeutic resistance of tumor cells. TRAIL (tumor-necrosis-factor-related apoptosis inducing ligand; http://en.wikipedia.org/wiki/TRAIL) is involved with induction of apoptosis in tumor cells thereby represents an attractive target for the development of anticancer therapy. A type II transmembrane protein and a member of tumor necrosis factor family, TRAIL is expressed in wide range of tissues. Even though it is a membrane protein, TRAIL is also expressed in soluble form. Induction of TRAIL-mediated apoptosis involves binding of TRAIL with its receptor TR1 (also known as death receptor 4, D R4) and TR2 (DR5). TRAIL also binds with two other receptors TR3 and TR4. Unlike TR1 and TR2, these receptors have incomplete death domains. Elevated expression of TR3 and TR4 in normal cells is thereby suggested to protect normal cells from TRAIL-induced death signaling. Induction of apoptotic signaling involves binding of TRAIL to DR4 or DR5. This results in homotrimerization and activation of receptors, enabling the receptors’ death domain to recruit the adaptor protein Fas-associated death domain along with pro-caspase- 8 and pro-caspase- 10. These all together then form the multi-protein death-inducing signaling complex, (DISC). Inside the DISC procaspeses become autoactivated and become caspase-8/10. Activated caspase-8/10 then activates downstream effector caspase-3 or caspase-7. Finally, cleavage of downstream substrates by effector caspases results in DNA fragmentation leading to apoptosis.
R4) and TR2 (DR5). TRAIL also binds with two other receptors TR3 and TR4. Unlike TR1 and TR2, these receptors have incomplete death domains. Elevated expression of TR3 and TR4 in normal cells is thereby suggested to protect normal cells from TRAIL-induced death signaling. Induction of apoptotic signaling involves binding of TRAIL to DR4 or DR5. This results in homotrimerization and activation of receptors, enabling the receptors’ death domain to recruit the adaptor protein Fas-associated death domain along with pro-caspase- 8 and pro-caspase- 10. These all together then form the multi-protein death-inducing signaling complex, (DISC). Inside the DISC procaspeses become autoactivated and become caspase-8/10. Activated caspase-8/10 then activates downstream effector caspase-3 or caspase-7. Finally, cleavage of downstream substrates by effector caspases results in DNA fragmentation leading to apoptosis.
Several studies assessed efficacy of TRAIL-based therapies in cancer as a single agent or in combination with other anti-cancer agents. In monotherapy, monoclonal antibodies conatumumab, AMG 655, CS-1008, dulanermin, exatumumab, and mapatumumab exhibited responses in follicular lymphoma, lung adenocarcinoma and in a rare form cancer synovial sarcoma with minimal toxicity. In addition, efficacy of TRAIL-monoclonal antibodies has also been evaluated in combination with anti-cancer agents such as paclitaxel, cisplatin, carboplatin, gemcitabine, histone deacetylase inhibitors, proteosome inhibitors, and kinase inhibitors. However, several cases of adverse effects and toxicities were reported in these studies. In addition, recent TRAIL-based therapies are expensive to produce for clinical applications and may also be limited by endogenous TRAIL level as well as dependency on tumor suppressor gene p53 as a regulator of TRAIL gene transcription. Therefore, studies are required to overcome the shortcoming of current TRAIL-based therapies.
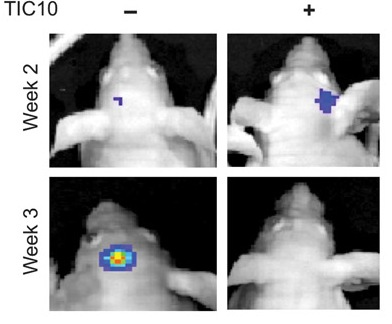
A preclinical study published recently in Science Translational Medicine (Allen et al., 2013) reported that small-molecule drug TRAIL-inducing compound 10 (TIC10) can trigger tumor cell death in a variety of cancers including breast, colon, lung, and also gliobalstoma, a type of brain tumor that is very difficult to treat. The anti-tumor activity of TIC10 is mediated by the activation of TRAIL in Foxo3a dependent manner. In their study, Allen et al. observed significant tumor regression in human colon, triple-negative breast, non-small cell lung, and brain cancer xenograft-bearing mice following single-dose treatment of TIC10. Inactivation of both phosphoinositide 3-kinase/Akt (PI3-K/Akt) and mito gen-activated protein kinase (MAPK) pathways was noted following TIC10 treatment in this in vivo study that resulted in translocation of the transcription factor Foxo3a into the nucleus, where Foxo3a induced expression of TRAIL gene to activate apoptosis.
gen-activated protein kinase (MAPK) pathways was noted following TIC10 treatment in this in vivo study that resulted in translocation of the transcription factor Foxo3a into the nucleus, where Foxo3a induced expression of TRAIL gene to activate apoptosis.
In the pre-clinical study, small-molecule drug TIC10 showed potent anti-tumor activity with limited toxicity through sustained induction of TRAIL even in refractory brain malignancy in a p53-independent manner. This revives the hope of TRAIL-based therapies in cancer. However, clinical trials need to be conducted first in order to confirm safety and efficacy of TIC10 before it can ultimately be used within the clinical setting.
References:
1. Allen JE, Krigsfeld G, Mayes PA, et al. Dual Inactivation of Akt and ERK by TIC10 Signals Foxo3a Nuclear Translocation, TRAIL Gene Induction, and Potent Antitumor Effects. Sci Transl Med. 2013;5(171):171ra117.
2. Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol Cancer Ther. 2012;11(1):3-13.
3. Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2012.