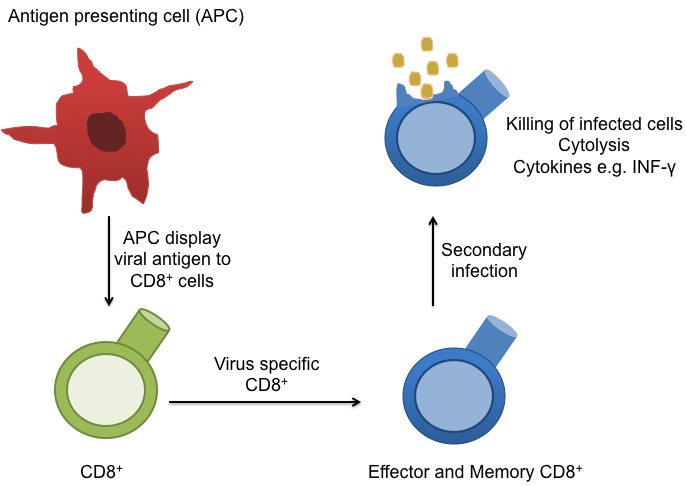
Memory CD8+ T cells continually  circulate in the blood, lymph, and secondary lymphatic organs as they patrol for the presence of secondary infections. Recently, a class of effector memory CD8+ T cells has also been shown to migrate to and reside long-term in non-lymphoid tissues. These tissue resident memory T cells can quickly respond to tissue infections with their cognate pathogen. CD8+ T cells mainly function in killing of infected cells though cytolysis and by producing effector cytokines, including IFN-gamma to activate other immune cells. In a recent article in Nature Immunology, Schenkel et. al demonstrate an additional function for these tissue resident memory CD8+ T cells: as major producers of chemokines that recruit circulating memory T cell forces to the site of infection.
circulate in the blood, lymph, and secondary lymphatic organs as they patrol for the presence of secondary infections. Recently, a class of effector memory CD8+ T cells has also been shown to migrate to and reside long-term in non-lymphoid tissues. These tissue resident memory T cells can quickly respond to tissue infections with their cognate pathogen. CD8+ T cells mainly function in killing of infected cells though cytolysis and by producing effector cytokines, including IFN-gamma to activate other immune cells. In a recent article in Nature Immunology, Schenkel et. al demonstrate an additional function for these tissue resident memory CD8+ T cells: as major producers of chemokines that recruit circulating memory T cell forces to the site of infection.
In this study, Lymphocytic choriomeningitis virus (LCMV) was injected intraperitoneally into female mice that had been adoptively transferred with naïve TCR transgenic CD8+ T cells specific for the gp33 epitope expressed by LCMV. Two months later, mice were transcervically infected with a Vaccinia virus strain engineered to express gp33 (VV-gp33) and the kinetics of the CD8 T cell recall response in the mucosal tissues of the female reproductive tract were assessed. Gp33-specific tissue resident memory CD8+ T cells were required for the rapid (within 2 days) recruitment of additional circulating gp33-specific T cells into the tissues. However, if the secondary infection was with a Vaccinia virus strain expressing a different antigen (OVA), then relatively few memory T cells were seen accumulating in the reproductive tract tissue. This specificity was also seen when gp33 versus OVA peptides were transcervically injected into the tissues instead of infection with VV.
To derive the mechanisms underlying the recruitment of T cells to infected tissue sites, chemokine expression was assessed. CXCL9 as well as multiple other chemokines were found to be rapidly induced in various cells residing in the reproductive tissues including endothelial cells, tissue dendritic cells, and memory CD8+ T cells. Production of IFN-gamma by the tissue resident memory CD8+ T cells was required for both recruitment of additional memory CD8+ T cells to the site as well as CXCL9 production by endothelial cells.
Antigen presenting cells as well as activated T cells produce copious amounts of IFN-gamma and it is expected that IFN-gamma production would elicit CXCL9 expression as the alternate name of CXCL9 is monokine induced by IFN-gamma (MIG). In this study however, it was production of IFN-gamma by the antigen-specific (gp33) tissue resident memory CD8+ T cells that was critical in the expression of chemokines such as CXCL9 and further recruitment of additional memory CD8+ T cells into the infected tissues. Antigen presenting cells alone were not as productive in this process because when the secondary infection was instead performed with an OVA-antigen expressing vaccinia virus, significant numbers of OVA-specific T cells were not recruited to the infected tissues.
Thus, this study demonstrated that antigen-specific CD8+ memory T cells that reside in tissues function to significantly amplify innate immune alarms to secondary infections by recruiting additional circulating CD8+ memory T cells to the infected site.
Further Reading:
Sensing and alarm function of resident memory CD8(+) T cells. Schenkel JM, Fraser KA, Vezys V, Masopust D. Nat Immunol. 2013 May;14(5):509-13. doi: 10.1038/ni.2568. Epub 2013 Mar 31.
Hidden memories: frontline memory T cells and early pathogen interception. Masopust D, Picker LJ. J Immunol. 2012 Jun 15;188(12):5811-7. doi: 10.4049/jimmunol.1102695.
Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A. J Immunol. 2004 Jun 15;172(12):7417-24.