Breast cancer is the most common cancer in women and the second-leading cause of cancer-related death in women worldwide. Despite progresses in the treatment of early stage breast cancer, approximately one third of patients will develop metastatic breast cancer (MBC). According to the National Cancer Institute, in USA, the estimated new cases and deaths from breast cancer in 2013 would be 232,340 and 39,620 respectively.
Approximately 20%–30% of breast cancers exhibit increased expression of human epidermal growth factor receptor 2 (HER-2/neu) caused by amplification of the erb-B2 oncogene. Breast cancers with elevated HER-2 expression are known as HER2-positive cancers. HER-2-positive breast cancers are more aggressive than other breast cancers. Patients with these tumors have a poorer prognosis and decreased chance of survival compared with patients whose tumors do not overexpress HER-2.
 HER-2 is a 185-kDa orphan transmembrane receptor tyrosine kinase. Dimerization of HER-2 with ligand- bound HER-3 or HER-4 receptor activates signaling pathways inside the cell. Activated HER-2 signaling stimulates cell proliferation and survival via activation of the MAPK and PI3K/Akt/mTOR pathways. Collectively these signaling pathways result in uncontrolled growth of the tumor. Several studies suggested that the overexpression/amplification of HER-2 may lead to the development and progression of pre-malignant breast disease and also tumor metastasis. Therefore, the association of HER-2 in breast cancer as well as its involvement in tumor aggressiveness makes this receptor an appropriate target for tumor-specific therapies. Several strategies have been developed to inhibit HER-2 signaling. These include a tyrosine kinase inhibitor called lapatinib and a recombinant humanized monoclonal antibody called trastuzumab (Herceptin®). In this post I will focus only on trastuzumab mediated therapy in breast cancer. Trastuzumab binds to the extracellular domain of the HER-2 receptor. This inhibits HER-2 signaling via MAPK and PI3K/Akt cascades. In addition, trastuzumab binding also increases membrane localization of the tumor suppressor gene phosphatase and tensin homolog (PTEN), and inhibitor of the PI3K/Aktpathway.
HER-2 is a 185-kDa orphan transmembrane receptor tyrosine kinase. Dimerization of HER-2 with ligand- bound HER-3 or HER-4 receptor activates signaling pathways inside the cell. Activated HER-2 signaling stimulates cell proliferation and survival via activation of the MAPK and PI3K/Akt/mTOR pathways. Collectively these signaling pathways result in uncontrolled growth of the tumor. Several studies suggested that the overexpression/amplification of HER-2 may lead to the development and progression of pre-malignant breast disease and also tumor metastasis. Therefore, the association of HER-2 in breast cancer as well as its involvement in tumor aggressiveness makes this receptor an appropriate target for tumor-specific therapies. Several strategies have been developed to inhibit HER-2 signaling. These include a tyrosine kinase inhibitor called lapatinib and a recombinant humanized monoclonal antibody called trastuzumab (Herceptin®). In this post I will focus only on trastuzumab mediated therapy in breast cancer. Trastuzumab binds to the extracellular domain of the HER-2 receptor. This inhibits HER-2 signaling via MAPK and PI3K/Akt cascades. In addition, trastuzumab binding also increases membrane localization of the tumor suppressor gene phosphatase and tensin homolog (PTEN), and inhibitor of the PI3K/Aktpathway.
In 1998 trastuzumab was approved for thetreatment of metastatic breast cancer (MBC), and in 2006 for the adjuvant treatment of HER2-overexpressing breast cancer. In early-stage breast cancer, treatment with trastuzumab and a neoadjuvant chemotherapy substantially improves overall survival (OS) and reduces the risk of recurrence, both by 33%. In MBC, trastuzumab treatment in combination with chemotherapy increases the time to progression of the disease by 49% and improves OS by 20%.
However, even though trastuzumab treatment substantially improves outcomes in both early-stage and MBC, both de novo and acquired resistance after initial response was observed. It is suggested that most patients with HER2-positive MBC will eventually develop resistance and have disease progression following trastuzumab treatment.
Several studies reported involvement of multiple factors in resistance to HER2-targeted therapy. These include hindrance to HER-2-trastuzumab binding, signaling through alternative pathways (for e.g. insulin-like growth factor receptor 1, vascular endothelial growth factor receptor) upregulation of signaling pathways downstream of HER-2, increased expression of heat shock protein 90 (HSP90), loss of PTEN and thereby constitutive activation of the PI3K/Akt pathway, and failure to induce an appropriate immune response.
To overcome transtuzumab-resistance, various treatment strategies have been developed. One strategy involves continuation of transtuzumab treatment in combination with a chemotherapeutic agent. In multiple pre-clinical and clinical studies, combination of 
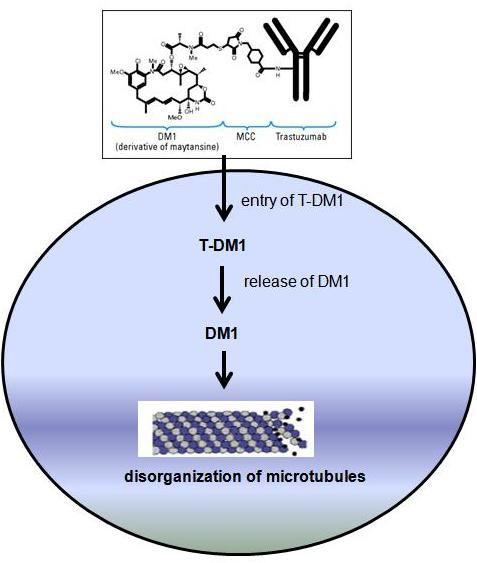
A new strategy to increase efficacy of trastuzumab has also been developed using antibody-drug conjugate (ADC) technology. The antibody-drug conjugate trantuzumab emtansine (T-DM1, Kadcyla) is consist of trastuzumab bound to maytansinoid (or DM1, a potent microtubule inhibitor) through a nonreducible thioether linkage. T-DM1 binds to HER-2 positive tumor cells and thought to inhibit HER-2 signaling. This ADC also induces body’s immune response to attack cancer cells. Once inside the tumor cells, T-DM1 is designed to kill tumor cells by releasing DM1 which is a potent inhibitor of microtubule assembly, thereby causing cell death inside the cells.
In in vitro and preclinical studies T-DM1 inhibited growth of breast cancer cells which are cross-resistant to trastuzumab. T-DM1 was found well tolerated in phase I clinical study of breast cancer patients who had disease progression with earlier trastuzumab based treatment. In phase II study, increased progression-free survival (PFS) was observed in patients treated with T-DM1 compared to trastuzumab plus doecetaxel treatment. A clinical study published by Verma et al. (2012) reported that T-DM1 significantly prolonged PFS and OS in patients with HER-2 positive MBC previously treated with trastuzumab and a taxane. The most common side effects of T-DM1 treatment include low platelet count, low RBC count, nerve problems, and tiredness. On the basis of clinical efficacy of T-DM1 observed in phase I and II trials, a multicenter phase III trial (also known as EMILIA trial) was performed. This trial also observed increased PFS, reduction of risk of death, and fewer adverse events in T-DM1 treated patients compared to capecitabine plus lapatinib treatment (another first-line treatment option for HER-2positive MBC).
On February 22nd, 2013, the US food and drug administration (FDA) approved T-DM1 (Kadcyla) for the treatment of HER-2 positive MBC that has progressed following treatment with trastuzumab and a taxane.
Suggested reading:
[1] M.F. Barginear, V. John, D.R. Budman, Trastuzumab-DM1: A Clinical Update of the Novel Antibody-Drug Conjugate for HER2-Overexpressing Breast Cancer, Mol Med, 18 (2013) 1473-1479.
[2] M. Barok, M. Tanner, K. Köninki, J. Isola, Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo, Breast Cancer Res, 13 (2011) R46.
[3] M.S. Mohd Sharial, J. Crown, B.T. Hennessy, Overcoming resistance and restoring sensitivity to HER2-targeted therapies in breast cancer, Ann Oncol, 23 (2012) 3007-3016.
[4] S. Verma, D. Miles, L. Gianni, I.E. Krop, M. Welslau, J. Baselga, M. Pegram, D.Y. Oh, V. Diéras, E. Guardino, L. Fang, M.W. Lu, S. Olsen, K. Blackwell, E.S. Group, Trastuzumab emtansine for HER2-positive advanced breast cancer, N Engl J Med, 367 (2012) 1783-1791.
[5]http://www.cancer.gov/cancertopics/understandingcancer/targetedtherapies/breastcancer_htmlcourse/page3