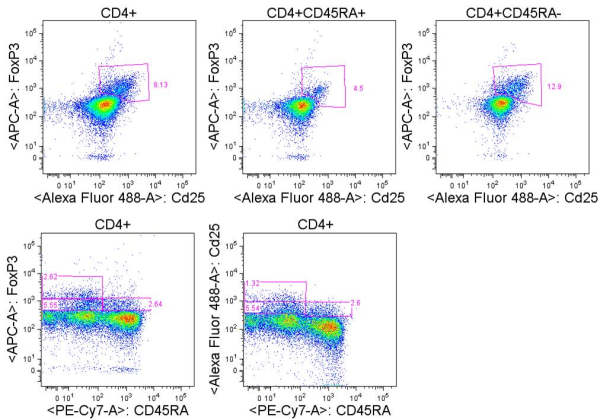
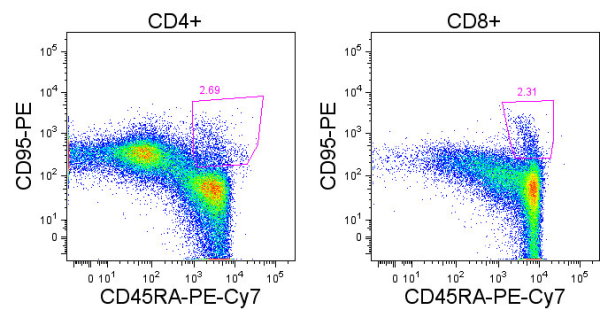
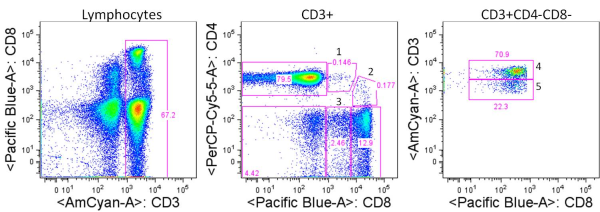
Human T cells are generally analyzed for expression of CD4 and CD8 to classify them as either of these two major classes of effector T cells. But flow cytometry analysis of PBMCs stained with antibodies targeting CD3, CD4, and CD8 reveals several other populations with varying expression of these three markers. So what are they?
For the sake of this discussion, I will refer to the largest typical single positive populations of CD3+CD4+ and CD3+CD8+ T cells as CD4high and CD8 high, respectively.
These are some other T cell populations that have been observed in human PBMC:
CD8lowCD4high (1): Populations of CD4 T cells that express CD8. This population is likely heterogeneous, and compared with the CD4high population, includes a higher proportion of effector memory and terminally differentiated effector CD4 T cells that have re-expressed CD8. CD8 is expressed as a heterodimer of either α/α, α/β, or β/β, and this population has been noted to be primarily CD8α/α. Work by Zloza et. al, identified that up to 50% of these cells can be NKT cells, including invariant CD3+6B11+ NKT cells and non-invariant CD3+CD16/56+ NKT cells. Of note, NKT cells may also be present at low frequencies in CD4+CD8+, CD4–CD8–, CD4 or CD8 single positive populations.
CD4lowCD8high (2): Populations of CD8+ T cells, of the primarily CD8α/β type that express CD4. This population can be further subdivided into two groups: CD4dimCD8high and CD4medCD8high. Studies have shown that expression of CD4 on these CD8+ T cells is functional and inducible by stimulations such as anti-CD3/CD28. These cells express markers of activated T cells and exhibit a higher frequency of memory cells (CD45RA–) as compared with typical CD8high cells.
CD8low (3): These cells express CD8 at lower levels compared with CD8 high populations, are negative for CD4 expression, and can express higher levels of CD3. Trautmann et. al, describe the frequency of CD8 low cells as being from 0.2%-7% of CD8 T cells in healthy donors and described these cells as populations of oligoclonal cytotoxic terminally differentiated effector CD8 T cells (CD45RA+CD62L–).

CD4neg CD8neg CD3high (4): CD4 and CD8 double negative cells that express high levels of CD3 compared with CD4 high and CD8 high populations. This fraction has been shown to contain largely the TCRγ/δ T cell subset although γ/δ T cells can express and the CD8α and/or the CD8β chains.
CD4neg CD8neg CD3pos (5): This fraction has been shown to largely contain heterogeneously differentiated TCRα/β T cell subsets including regulatory T cells. The expression of CD3 on this subset is lower than that of the CD4neg CD8neg CD3high subset containing γ/δ T cells, although γ/δ T cells may be present in this population as well.
An important thing to note is that characterizations of these populations are generalizations and individuals have been shown to have aberrant profiles compared with these. Other populations have been described such as CD4high CD8high double positive cells which may be primarily effector memory T cells but here I have focused on those populations I see most frequently. In summary, careful gating and analyses of each of these populations is necessary, as these are not only functionally unique subsets, but each population appears to be heterogeneous and also contain varying percentages of NKT cells.
Further Reading:
CD4(+)CD8(dim) T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Suni MA, Ghanekar SA, Houck DW, Maecker HT, Wormsley SB, Picker LJ, Moss RB, Maino VC. Eur J Immunol. 2001 Aug;31(8):2512-20.
Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. Zloza A. and Al-Harthi, L. Journal of Leukocyte Biology. J Leukoc Biol. 2006 Jan;79(1):4-6.
Characterization of circulating CD4+ CD8+ lymphocytes in healthy individuals prompted by identification of a blood donor with a markedly elevated level of CD4+ CD8+ lymphocytes. Prince HE, Golding J, York J. Clin Diagn Lab Immunol. 1994 Sep;1(5):597-605.
Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Immunology. 2001;103: 270-280.
Human CD8 T cells of the peripheral blood contain a low CD8 expressing cytotoxic/effector subpopulation. Trautmann A, Rückert B, Schmid-Grendelmeier P, Niederer E, Bröcker EB, Blaser K, Akdis CA. Immunology. 2003 Mar;108(3):305-12.
CD3 bright lymphocyte population reveal gammadelta T cells. Lambert C, Genin C. Cytometry B Clin Cytom. 2004 Sep;61(1):45-53.
Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A. Blood. 2005 Apr 1;105(7):2828-35.
Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. Nascimbeni M, Pol S, Saunier B. PLoS One. 2011;6(5):e20145.
CD4+ CD8+ double positive (DP) T cells in health and disease. Parel Y, Chizzolini C. Autoimmun Rev. 2004 Mar;3(3):215-20.

 Significant steps forward are being made in immunotherapeutic approaches for treatment of cancer. Over the past few years, two cancer immunotherapeutics were FDA approved. In 2010, Dendreon Corporation’s Provenge, an autologous cellular vaccine, was approved for hormone refractory metastatic prostate cancer. In 2011, Bristol-Myers Squibb’s anti-CTLA4 antibody Ipilimumab, was FDA approved for late stage melanoma. Recent promising clinical trial results indicate several additional immune modulating therapies are likely to join this prestigious list in the coming years. In addition, combinations of immune therapies with chemotherapeutics are being tested in clinical trials. In light of this, it is important to know how chemotherapies interact with the immune system, in order to best generate synergistic effects.
Significant steps forward are being made in immunotherapeutic approaches for treatment of cancer. Over the past few years, two cancer immunotherapeutics were FDA approved. In 2010, Dendreon Corporation’s Provenge, an autologous cellular vaccine, was approved for hormone refractory metastatic prostate cancer. In 2011, Bristol-Myers Squibb’s anti-CTLA4 antibody Ipilimumab, was FDA approved for late stage melanoma. Recent promising clinical trial results indicate several additional immune modulating therapies are likely to join this prestigious list in the coming years. In addition, combinations of immune therapies with chemotherapeutics are being tested in clinical trials. In light of this, it is important to know how chemotherapies interact with the immune system, in order to best generate synergistic effects.
 96-well Plates: Different types of 96-well plates are available for different assay types. There are various surface coatings including tissue-culture treated polystyrene for cell cultures, uncoated, and others. Plates can have various plate bottom geometries and optical characteristics. For instance there are black plates available for light-sensitive assays. For protocols involving volumes larger then 250ul, there are deep-well plates that carry a 2ml volume per well.
96-well Plates: Different types of 96-well plates are available for different assay types. There are various surface coatings including tissue-culture treated polystyrene for cell cultures, uncoated, and others. Plates can have various plate bottom geometries and optical characteristics. For instance there are black plates available for light-sensitive assays. For protocols involving volumes larger then 250ul, there are deep-well plates that carry a 2ml volume per well. Multichannel Vacuums: Companies such as V&P Scientific offer a multitude of multichannel vacuum manifolds that fit plates of different depths for removing supernatant from wells via vacuum apparatus. Often these will be the proper length such that they don’t touch the well bottom and work well with removing buffers from centrifuged PBMC cell cultures, such as during washing steps for flow-cytometry.
Multichannel Vacuums: Companies such as V&P Scientific offer a multitude of multichannel vacuum manifolds that fit plates of different depths for removing supernatant from wells via vacuum apparatus. Often these will be the proper length such that they don’t touch the well bottom and work well with removing buffers from centrifuged PBMC cell cultures, such as during washing steps for flow-cytometry.