Klotho is a type I transmembrane protein, expressed in the brain, kidneys and reproductive organs; it is named after “Clotho”, a goddess from Greek mythology who “spins the thread of life” (the length of the threat is determinant of how long a certain individual will live). This denomination is due to the direct positive correlation between Klotho’s expression levels and life span/anti-aging phenotypes.
Klotho protein exists in two forms, membrane Klotho and secreted Klotho, and each form is associated with distinct functions. Some of Klotho’s age-suppressing functions include regulation of fibroblast growth factor-23 (FGF23) signaling, inhibition of insulin/insulin-like growth factor (IGF-1) and Wnt signaling pathways, and regulation of multiple cell-surface ion channels and growth factor receptors.
In contrast to other organ systems, the downstream effects of Klotho have not been as extensively studied within the central nervous system (CNS). This is surprising, considering that not only Klotho is present in serum and cerebrospinal fluid (CSF), but also is highly expressed in the hippocampus, choroid plexus and neurons. Disruption of the myelin sheath, either by activated pro-inflammatory cells or by protein defection within the oligodendrocytes has been previously described in aging brain, but the underlying factor stimulating the disruption was not clear. However, In 2008, Abraham’s group reported the significance of Klotho in age-related cognitive decline (ARCD), showing reduced expression of Klotho in regions of brain white matter as a function of age.
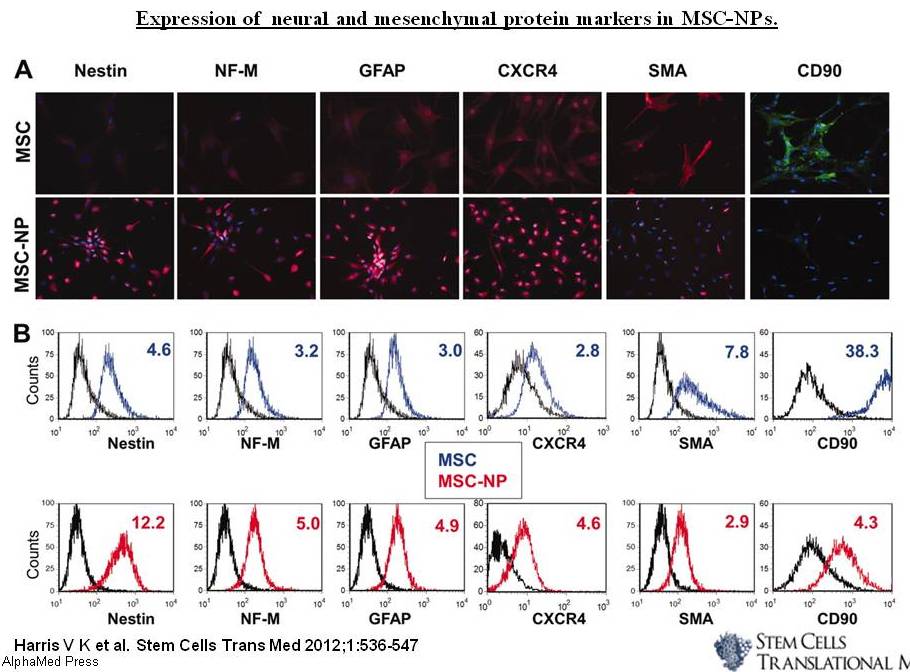
In January 2013, Abraham’s group reported their new findings regarding the effects of Klotho in oligodendrocyte maturation and CNS myelination, and its relation to ARCD. They showed Klotho‘s role in inducing oligodendrocytic progenitor cells (OPCs) maturation, by enhancing the expression of myelin proteins, such as myelin-associated glycoprotein (MAG), myelin basic protein (MBP), oligodendrocyte-specific protein (OSP/Claudin11), and proteolipid protein (PLP). Based on their in vivo studies, the loss of Klotho expression is correlated to defects in myelin that result in similar progressive axonal degeneration observed in hypomyelinating and demyelinating diseases, such as multiple sclerosis (MS).
Previous studies have shown Klotho’s role in facilitating removal of reactive oxygen species (ROS) and increasing resistance to oxidative stress. Furthermore, Nagai’s team observed impaired cognitive function in Klotho-deficient mice, as well as improved cognition upon treatment with the α-tocopherol antioxidant. Thus, Abraham’s group concluded that Klotho protein may function as a neuroprotective factor in the CNS through its antioxidative stress effect.
Together, these results provide strong evidence for Klotho protein as a key player, not only in myelin maintenance, but also in supporting oligodendrocyte and OPC function in the CNS; this makes Klotho an important member of the family of proteins responsible for neuron-oligodendrocyte communication. Abraham’s group hypothesized that downregulation of Klotho may be accountable for the observed white matter myelin degeneration-mediated ARCD, hence increasing Klotho protein expression can potentially prevent damage/protect myelin in the aging brain.
Abraham’s new findings are an exciting and important initial step towards development of new neuroprotective therapeutic strategies, such as induction of endogenous remyelination, for treatment of CNS diseases characterized by oligodendrocyte cell loss, such as MS and Schizophrenia. Additionally, early defects of insulin/IGF-1 receptor signaling in Alzheimer’s disease (AD), including the deficit of glucose metabolism that anticipates cognitive decline, may be partially due to deficiencies in Klotho levels. Further investigation on the precise mechanisms involved in Klotho’s regulation within the CNS seems promising for the future of neurodegenerative disease therapy.
Further Readings:
The Antiaging Protein Klotho Enhances Oligodendrocyte Maturation and Myelination of the CNS
The Putative Role of The Antiageing Protein Klotho in Cardiovascular and Renal Disease




 536-47, Figure 3.jpg)