In a previous posting, I discussed the use of T cell activation markers as a strategy for assessing the function of T cells from human peripheral blood mononuclear cells (PBMC). Following T cell receptor (TCR) activation, T cells will express a series of activation markers that include chemokine and cytokine receptors, adhesion molecules, co-stimulatory molecules, and MHC-class II proteins. Understanding what these activation markers are, when they are expressed, and their role in T cell function during normal responses and disease states is important when selecting markers for assessing T cell biology for studies on human PBMC.
In the previous posting, I discussed two immediate early activation markers for assessing the activation status of human PBMC T cells: CD69 and CD40L. In this article, the second in this series, I will discuss two additional mid-early T cell activation markers that can be assessed by flow cytometry: CD71 and CD95.
CD71 (TFRC, Transferrin Receptor, TfR) is a cell surface iron transport receptor that is upregulated in proliferating cells by 24-48 hours following T cell activation and expression continues to rise and is maintained for several days. Thus CD71 can be considered a mid-early activation marker as compared with late activation markers that are not appreciably upregulated until even later time points. CD71 has been shown to associate with the TCRz chain and ZAP70 and may participate in TCR signaling, and is an essential factor for proliferating T cells.
The inability of CD71 to be upregulated following TCR activation may be associated with T cell dysfunction. As was similarly discussed for CD69, Critchley-Thorne et. al, 2007 showed that PBMC T cells from metastatic melanoma patients had reduced CD71 upregulation compared with healthy controls, and this corresponded with multiple other functional defects in T cells from these patients. Thus CD71 may be aberrantly expressed by T cells in human disease.
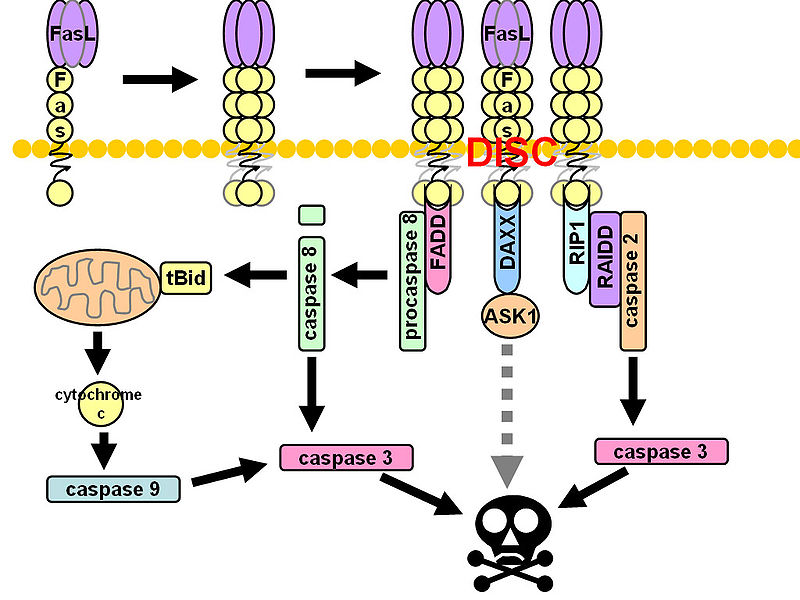
 CD95 (Fas, APO-1, TNFRSF6) is a member of the TNF-receptor superfamily and is best known for its role in mediating activation-induced cell death in activated T cells following binding to its ligand, CD95L/FasL induced on antigen-presenting cells (APCs). However, CD95 can also play additional, non-apoptotic roles in the modulation of T cell function. CD95 ligation has been shown to inhibit TCR signaling and activation of naïve T cells. However, this negative co-stimulatory effect appears to be dose-dependent, as low doses of CD95 agonists had the opposite effect and strongly promoted activation and proliferation of T cells. Like CD71, CD95 expression can be detected by 24 hours following T cell activation and continues to increase over the course of several days.
CD95 (Fas, APO-1, TNFRSF6) is a member of the TNF-receptor superfamily and is best known for its role in mediating activation-induced cell death in activated T cells following binding to its ligand, CD95L/FasL induced on antigen-presenting cells (APCs). However, CD95 can also play additional, non-apoptotic roles in the modulation of T cell function. CD95 ligation has been shown to inhibit TCR signaling and activation of naïve T cells. However, this negative co-stimulatory effect appears to be dose-dependent, as low doses of CD95 agonists had the opposite effect and strongly promoted activation and proliferation of T cells. Like CD71, CD95 expression can be detected by 24 hours following T cell activation and continues to increase over the course of several days.
Due to its differential roles in regulation of T cell apoptosis and activation, dysregulated expression of CD95 or its ligand CD95L could be avenues for T cell dysfunction in various human diseases. Indeed, Strauss et. al, showed that regulation of CD95L expression may play a role in immune evasion during viral infections. CD95L was upregulated in HIV-infected APCs, and led to suppressed T cell activation. Interferons are known to enhance CD95 expression, and our group (Critchley-Thorne et. al, 2009) has shown reduced upregulation of CD95 in PBMC T cells from breast cancer patients following T cell activation in the presence of interferons, indicating the lack of full T cell activation under these conditions.
Thus both CD71 and CD95 are upregulated in the mid-early phase of T cell activation and dysfunctional expression may be useful measures of T cell dysfunction in various disease states. Thus, these may be useful markers when assessing the phenotype and function of human PBMCs.
Additional Reading:
Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. J Immunol Methods. 2004 Oct;293(1-2):127-42.
Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Biselli R, Matricardi PM, D’Amelio R, Fattorossi A. Scand J Immunol. 1992 Apr;35(4):439-47.
Surface markers of lymphocyte activation and markers of cell proliferation. Shipkova M, Wieland E. Clin Chim Acta. 2012 Sep 8;413(17-18):1338-49.
Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, Fiorentini S, Peroni L, Fallacara F, Dima F, Balsari A, Turano A. Cytometry. 1997 Jan 1;27(1):71-6.
Transferrin receptor induces tyrosine phosphorylation in T cells and is physically associated with the TCR zeta-chain. Salmerón A, Borroto A, Fresno M, Crumpton MJ, Ley SC, Alarcón B. J Immunol. 1995 Feb 15;154(4):1675-83.
Transferrin synthesis by inducer T lymphocytes. Lum JB, Infante AJ, Makker DM, Yang F, Bowman BH. J Clin Invest. 1986 Mar;77(3):841-9.
Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. PLoS Med. 2007 May;4(5):e176.
Pro- and anti-apoptotic CD95 signaling in T cells. Paulsen M, Janssen O. Cell Commun Signal. 2011 Apr 8;9:7.
CD95 co-stimulation blocks activation of naive T cells by inhibiting T cell receptor signaling. Strauss G, Lindquist JA, Arhel N, Felder E, Karl S, Haas TL, Fulda S, Walczak H, Kirchhoff F, Debatin KM. J Exp Med 2009, 206:1379-1393.
Impaired interferon signaling is a common immune defect in human cancer. Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, Swetter SM, Carlson RW, Fisher GA, Koong A, Holmes S, Lee PP. Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9010-5.
*Image courtesy of http://en.wikipedia.org/wiki/Fas_ligand*