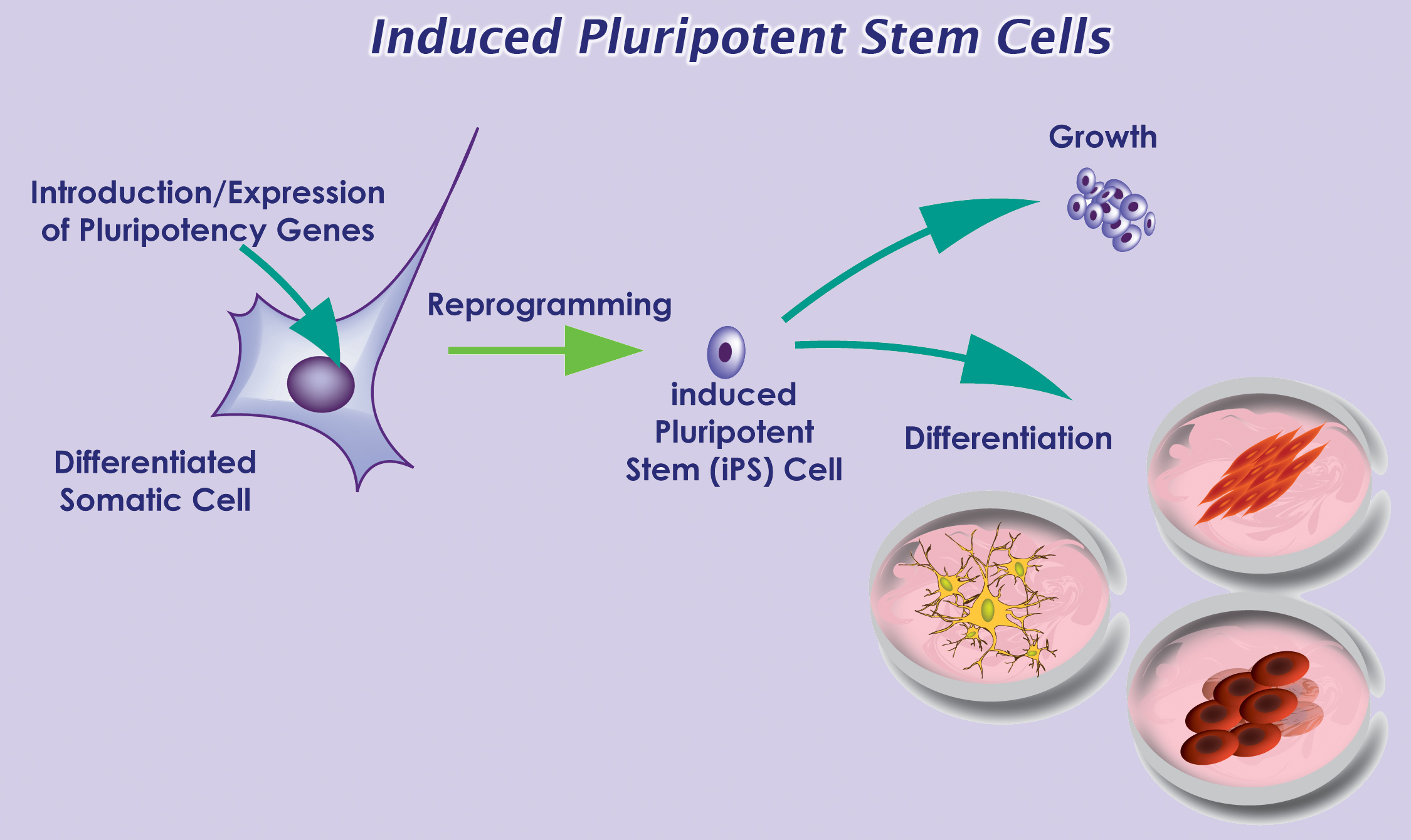
Last week, the International Society for Stem Cell Research (ISSCR) held its 11th Annual Meeting in Boston, MA. Over 3,000 stem cell researchers from around the world gathered to hear the leading experts share their research and perspectives in this fast-paced field. One interesting topic was recent advances in cell-replacement therapy for treatment of type 1 diabetes.
Diabetes mellitus is a metabolic disease that results from a failure in glucose regulation, often leading to severe hyperglycemia and tissue/organ damage. Pancreatic β-cells respond to high blood glucose levels by secreting insulin, which acts on other tissues to promote glucose uptake from the blood. Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing β-cells of the pancreas. The lack of insulin leads to increased blood and urine glucose. T1D is often fatal unless treated with exogenous administration of insulin daily and regular blood glucose monitoring for the patient’s entire life. However, this treatment does not match the effect of having endogenous β-cells. Thus, scientists in the field of regenerative medicine have focused on strategies for generation of β-cells for cell-replacement therapy.
Douglas Melton presented his lab’s  current progress on generation of functional β-cells from human embryonic stem cells (hESCs). Since 2006, D’Amour et al. demonstrated that they had developed a robust differentiation protocol to produce hESC-derived pancreatic endocrine cells capable of synthesizing insulin 1. However, the derived cells failed to secrete insulin appropriately in response to the addition of glucose, a required function of true β-cells. Thus, one focus of Melton’s group has been to identify the signals to generate functional β-cells. Pancreatic islets are complex structures consisting of multiple cell types, including the insulin-producing β-cells as well as endothelial cells in the surrounding blood vessels. Thus, endothelial signals are known to promote pancreatic development 2. Melton’s group found that co-culture of β-cells with endothelial cells promotes functional maturation of the hESC-derived β-cells. In addition, they recently identified another hormone, betatrophin, which is secreted by the liver and functions in promoting β-cell replication 3. Thus, increasing the levels of this hormone may generate more β-cells. Although various groups have demonstrated that large amounts of glucose-responsive, insulin-secreting β-cells can be generated in vitro, one concern that remains to be addressed is how the cells will be protected from an autoimmune attack once delivered to the patient. One potential strategy involves encapsulation of the β-cells into an immunoprotective device prior to delivery.
current progress on generation of functional β-cells from human embryonic stem cells (hESCs). Since 2006, D’Amour et al. demonstrated that they had developed a robust differentiation protocol to produce hESC-derived pancreatic endocrine cells capable of synthesizing insulin 1. However, the derived cells failed to secrete insulin appropriately in response to the addition of glucose, a required function of true β-cells. Thus, one focus of Melton’s group has been to identify the signals to generate functional β-cells. Pancreatic islets are complex structures consisting of multiple cell types, including the insulin-producing β-cells as well as endothelial cells in the surrounding blood vessels. Thus, endothelial signals are known to promote pancreatic development 2. Melton’s group found that co-culture of β-cells with endothelial cells promotes functional maturation of the hESC-derived β-cells. In addition, they recently identified another hormone, betatrophin, which is secreted by the liver and functions in promoting β-cell replication 3. Thus, increasing the levels of this hormone may generate more β-cells. Although various groups have demonstrated that large amounts of glucose-responsive, insulin-secreting β-cells can be generated in vitro, one concern that remains to be addressed is how the cells will be protected from an autoimmune attack once delivered to the patient. One potential strategy involves encapsulation of the β-cells into an immunoprotective device prior to delivery.
Other mature cells have also  been proposed as a source of new β-cells. Sarah Ferber presented her lab’s work on inducing liver cells to transdifferentiate into β-cells for autologous cell-replacement therapy. Transdifferentiation is the process by which one type of adult cell is directly converted into another type of cell. They used the transcription factor, pancreatic and duodenal homeobox gene 1 (PDX-1), and soluble factors to induce the developmental shift of adult human liver cells into functional insulin-producing cells 4. Not only did the transdifferentiated liver cells produce insulin, but they also released in a glucose-regulated manner. When transplanted into diabetic, immunodeficient mice, the cells ameliorated hyperglycemia over a 60-day period. Thus, PDX-1-induced transdifferentiated liver cells offer the potential to replace β-cells’ function in vivo. Furthermore, transplantation of autologous β-cells would circumvent a host versus graft immune response, as well as allow the patient to be the donor of his or her own insulin-producing cells.
been proposed as a source of new β-cells. Sarah Ferber presented her lab’s work on inducing liver cells to transdifferentiate into β-cells for autologous cell-replacement therapy. Transdifferentiation is the process by which one type of adult cell is directly converted into another type of cell. They used the transcription factor, pancreatic and duodenal homeobox gene 1 (PDX-1), and soluble factors to induce the developmental shift of adult human liver cells into functional insulin-producing cells 4. Not only did the transdifferentiated liver cells produce insulin, but they also released in a glucose-regulated manner. When transplanted into diabetic, immunodeficient mice, the cells ameliorated hyperglycemia over a 60-day period. Thus, PDX-1-induced transdifferentiated liver cells offer the potential to replace β-cells’ function in vivo. Furthermore, transplantation of autologous β-cells would circumvent a host versus graft immune response, as well as allow the patient to be the donor of his or her own insulin-producing cells.
In summary, Melton’s and Ferber’s presentations demonstrated the tremendous progress that has been made in the last decade to generate functional β-cells for use in treatment of T1D.
References
1 D’Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24, 1392-1401, doi:10.1038/nbt1259 (2006).
2 Nikolova, G. et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 10, 397-405, doi:10.1016/j.devcel.2006.01.015 (2006).
3 Yi, P., Park, J. S. & Melton, D. A. Betatrophin: A Hormone that Controls Pancreatic beta Cell Proliferation. Cell 153, 747-758, doi:10.1016/j.cell.2013.04.008 (2013).
4 Sapir, T. et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A 102, 7964-7969, doi:10.1073/pnas.0405277102 (2005).