Immunologists study many aspects regarding differentiation of T cells and function of T cell lineages. The results and interpretations from these studies always rely on the robustness of the experimental setup. A question that I posed to myself recently, when testing protocols for differentiation of naïve CD4+ T cells into various functional lineages (TH1, TH2, TH17, TREG), was whether or not CD4+ terminally differentiated effector memory (TEMRA) cells are still present in the population of “naïve” CD4+ T cells obtained following isolation from peripheral blood mononuclear cells (PBMC).
Following antigen exposure, CD4+ and CD8+ T cells undergo differentiation thorough various stages. While the exact path of differentiation remains under exploration, a current mainstream hypothesis is that naïve cells (TN) progress through central memory (TCM), then effector memory (TEM), then finally terminally differentiated effector memory (TEMRA) states. Expression of surface markers have been used to identify human T cells in these various states, including CD45RA, CD45RO, CCR7, CD62L, CD27, and CD28. After antigen exposure, naïve T cells, which are CD45RA+CD45RO–CCR7+CD62L+CD27+CD28+ lose expression of CD45RA and gain expression of CD45RO. As memory T cells progress from TCM to TEM cells, they additionally lose expression of CCR7, CD45RA+, CD27, and CD28. Finally, TEMRA cells regain expression of CD45RA, but remain identifiable from naïve T cells by their lack of CCR7, CD62L, CD27, and CD28 expression.
The function of CD4+ TEMRA cells parallels that of CD8+ TEMRA cells. These cells are cytolytic and express IFN-gamma after activation through their TCR or stimulation with PMA/ionomycin. CD4+ TEMRA cells also have shorter telomeres than naïve, TCM, and TEM populations, and lower homeostatic proliferation capacity.
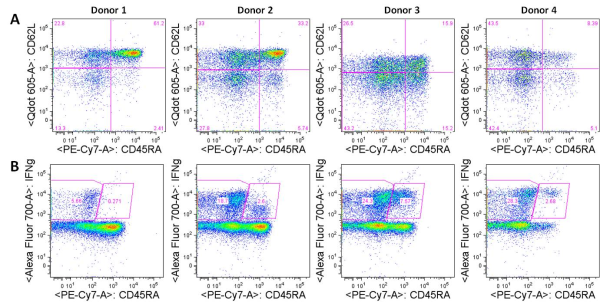
While TEMRA cells are well described for CD8+ T cells, they often are ignored as part of the CD4+ compartment. Despite the lack of attention that CD4+ TEMRA cells are given in the literature, I observe them quite frequently in human PBMC from healthy donors, on average being 4% of CD4+ T cells (range 0-15%) and 11% of CD4+CD45RA+ cells (range 0-40%). Additionally, the percentage of CD4+ TEMRA cells that I observe has a strong correlation with IFN-gamma production by CD4+CD45RA+ T cells from the same donor.

Figure: A. Expression of CD45RA vs. CD62L in human CD4+ PBMCs from four donors. CD4+ TEMRA cells are CD45RA+CD62L– (lower right quadrant). B. Expression of CD45RA vs. IFN-gamma in human CD4+ PBMCs from the same four donors stimulated with PMA/ionomycin. CD45RA+IFN-gamma+ cells are likely CD4+ TEMRA cells.
Considering CD4+ TEMRA cells are not only commonly present but highly functional, I wondered if they would be present in the population of “naïve” CD4+ T cells obtained following isolation from PBMC. If fluorescence-activated cell sorting (FACS) is used for cell isolation, then this is an easier issue to avoid as all of the necessary markers used to differentiate naïve CD4+ T cells from other cell subsets can be included in the marker staining panel. However, many researchers use commercially available magnetic bead-based kits or other similar methodologies to obtain a “naïve” CD4+ T cell population. Because there is no single marker that would isolate a naïve CD4 T cell from PBMC, negative selection kits for untouched isolation of naïve CD4+ T cells are commercially available as “one-step” kits. These are available from companies including Miltenyi Biotec, Stem Cell Technologies, and R&D Systems.
Analysis of the antigens negatively selected for by these kits revealed the following lists: Miltenyi Biotec: CD45RO, CD8, CD14, CD15, CD16, CD19, CD25, CD34, CD36, CD56, CD123, TCRγ/δ, HLA-DR, and CD235a (glycophorin A).
Stem Cell Technologies: CD45RO, CD8, CD14, CD16, CD19, CD20, CD36, CD56, CD66b, CD123, TCRγ/δ, and CD235a (glycophorin A).
Unfortunately, nothing in the literature indicated that any of these markers targeted removal of TEMRA cells, and the manufacturer’s data sheets only show that the final product obtained by using these kits are CD4+CD45RA+ cells. Technical support from both Miltenyi Biotec and Stem Cell Technologies came to the same conclusion: the CD4+ TEMRA cells are not removed.
The conclusion: Isolation of naïve CD4 cells without TEMRA cells may still be possible if this is necessary for your assays. Following usage of one of the above mentioned kits, positive selection for CCR7, CD62L, CD27, or CD28 can be tested.
The final question is whether these cells can affect the experimental results from for instance, studies on T-helper (TH) subset differentiation. While it is unknown if these cells themselves could differentiate into TH subtypes, they certainly can produce IFN-gamma in culture which inhibits TH subset differentiation along non-TH1 lineages, underscoring the necessity for the inclusion of anti-IFN-gamma antibodies when differentiating TH subtypes other than TH1.
Further reading:
eBiosciences Human CD & Other Cellular Antigens Chart
**Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Harari A, Vallelian F, Pantaleo G. Eur J Immunol. 2004 Dec;34(12):3525-33.
Phenotype and function of human T lymphocyte subsets: consensus and issues. Appay V, van Lier RA, Sallusto F, Roederer M. Cytometry A. 2008 Nov;73(11):975-83. doi: 10.1002/cyto.a.20643.
Phenotypic and functional profiling of CD4 T cell compartment in distinct populations of healthy adults with different antigenic exposure. Roetynck S, Olotu A, Simam J, Marsh K, Stockinger B, Urban B, Langhorne J. PLoS One. 2013;8(1):e55195. doi: 10.1371/journal.pone.0055195. Epub 2013 Jan 28.
Sensitive gene expression profiling of human T cell subsets reveals parallel post-thymic differentiation for CD4+ and CD8+ lineages. Appay V, Bosio A, Lokan S, Wiencek Y, Biervert C, Küsters D, Devevre E, Speiser D, Romero P, Rufer N, Leyvraz S. J Immunol. 2007 Dec 1;179(11):7406-14.
Characterization of CD4(+) CTLs ex vivo. Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, Grey P, Smith D, McMichael AJ, Cooper DA, Rowland-Jones SL, Kelleher AD. J Immunol. 2002 Jun 1;168(11):5954-8.
Altered proportions of naïve, central memory and terminally differentiated central memory subsets among CD4+ and CD8 + T cells expressing CD26 in patients with type 1 diabetes. Matteucci E, Ghimenti M, Di Beo S, Giampietro O. J Clin Immunol. 2011 Dec;31(6):977-84. doi: 10.1007/s10875-011-9573-z. Epub 2011 Sep 2.