Antibody based isolation kits for isolating immune cell populations have become a standard protocol in the toolbox of every immunologist over the last two decades. In fact, many new scientists are shocked to learn that lymphocytes used to be isolated from PBMCs and other tissue sources by filtering through nylon wool. How archaic! Here I will describe the various options cell isolation technologies available to biologists today.
FACS: Fluorescence Activated Cell Sorting
FACS is the most sophisticated way of isolating various cells of interest from your tissue source. You have the ability to incorporate up to 10 or so different fluorescent antibodies into your stain, which allows you to sort on cells of interest with exquisite precision and specificity. Another powerful tool is the ability of many FACS machines to do four-way sorts or even single-cell sorts.
However, sorting can be relatively time consuming, depending on your sample size and the percentage of cells of interest. Use of FACS machines are also fairly expensive, whether it be your laboratory’s investment in acquiring its own machine and committing to its maintenance or the hourly rates your institution’s core will charge you (averaging around $100 per hour in my experience).
Magnetic Antibody Based Cell Isolation
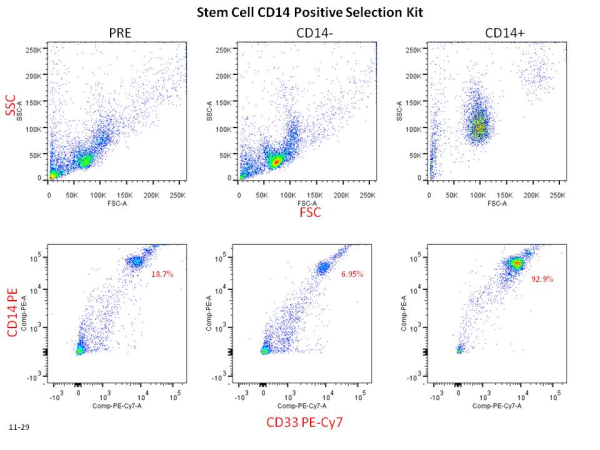
Cell separation reagents are available from the three main players in the cell isolation kit world: Stem Cell Technologies, Miltenyi Biotec MACS Technology, and Life Technologies Dynabeads. Though the technology varies slightly from company to company, they basically boil down to the same principles. Usually an antibody cocktail will bind either your cell of interest (positive selection) or your cells of non-interest (negative selection). After a short incubation the addition of magnetic nanoparticle beads to your cell mixture then binds the antibodies from the previous incubation. After another short incubation, cells can then be placed into the magnet purchased from the company. After a few minutes, the antibody bound cells will be drawn towards the magnet and the unbound cells can be collected. Bound cells can then be washed out and collected separately. This technology allows rapid and easy isolation of cell populations from bulk populations.
However, magnetic antibody based cell isolation involves some upfront investment in the purchasing of magnets (approaching $1000) and antibody kits (ranging from $300-$700). Because of this it is important to fully research which companies’ technology is right for you. I also highly recommend sampling the technology on some extra PBMCs you have if at all possible and finding an experienced colleague that can advise when you have questions.
RosetteSep Whole Blood Based Cell Isolation
RosetteSep kits from Stem Cell Technologies allow researchers to quickly isolate cells of interest directly from whole blood and without the investment in magnets. Furthermore it combines the Ficoll gradient isolation step with the isolation of specific target cells, making for an efficient and economical protocol. Instead of using magnetic nanoparticles, RosetteSep uses antibodies that conjugate directly to the RBCs in whole blood. When the blood is Ficolled the RBCs go to the bottom layer along with all the cells that you have targeted with antibody. Your top layer is left with untouched cells of your interest! Of course this protocol only works from whole blood, so it will not work on PBMCs or cells from other tissue sources.
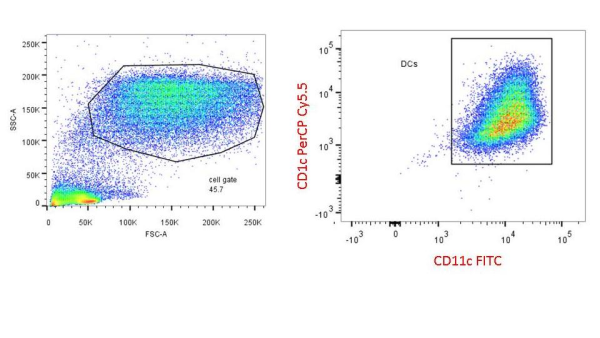
Keep in mind that both FACS and antibody based cell isolation require starting with a single cell suspension of cells. It is important to think about whether you want touched or untouched cells (positive or negative selection) for your downstream assays. I also highly recommend doing purity checks (see figure below) by flow cytometry as often as you can, especially when first adapting any isolation technology to your lab.

These powerful techniques allow for biologists to isolate a host of cells, including T cells, B cells, Monocytes, Stem Cells, and many more. In an upcoming post I will go into even further detail and how to choose the right technology for you, including some of the tips and tricks I have learned in my own experience
Further Reading:
Stem Cell Technologies: http://www.stemcell.com/en/Products/Product-Type/Cell-isolation-products.aspx
Life Technologies Dynabeads: http://www.invitrogen.com/site/us/en/home/brands/Product-Brand/Dynal/Dynabeads-Types-and-Uses.html
Miltenyi Biotec MACS Technology: https://www.miltenyibiotec.com/en/Products-and-Services/MACS-Cell-Separation.aspx
RosetteSep: http://www.stemcell.com/en/Products/Popular-Product-Lines/RosetteSep.aspx




 Colt Egelston is currently a post-doctoral fellow at the Beckman Research Institute of the City of Hope, in Duarte, CA. He received his Ph.D. from Rush University in Chicago and is interested in all things immunology.
Colt Egelston is currently a post-doctoral fellow at the Beckman Research Institute of the City of Hope, in Duarte, CA. He received his Ph.D. from Rush University in Chicago and is interested in all things immunology.
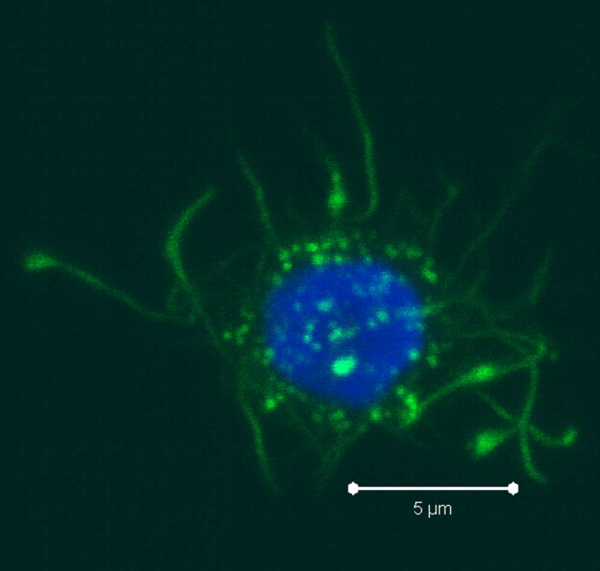
 PBMCs are not just a source of many different circulating immune cell types, but also a source of potential cells that one can generate in vitro. One excellent and long-standing example of this is the generation of dendritic cells (DCs) from monocytes. Monocyte derived DCs (mDCs) are an excellent tool for researchers to do immunological assays requiring a source of professional antigen presenting cells (APCs). While circulating B cells are capable of antigen presentation and T cell activation, they do not offer the robust response that DCs do. The generation of mDCs is a relatively simple protocol that anyone can do with just a source of PBMCs, a few important cytokines, and, of course, some media and incubator space. After this protocol, you will have obtained immature mDCs that can then be matured for use as APCs in your assay.
PBMCs are not just a source of many different circulating immune cell types, but also a source of potential cells that one can generate in vitro. One excellent and long-standing example of this is the generation of dendritic cells (DCs) from monocytes. Monocyte derived DCs (mDCs) are an excellent tool for researchers to do immunological assays requiring a source of professional antigen presenting cells (APCs). While circulating B cells are capable of antigen presentation and T cell activation, they do not offer the robust response that DCs do. The generation of mDCs is a relatively simple protocol that anyone can do with just a source of PBMCs, a few important cytokines, and, of course, some media and incubator space. After this protocol, you will have obtained immature mDCs that can then be matured for use as APCs in your assay. Once the culture period has finished, between 6-8 days, the mDCs can be collected. The exact day is not critical, as long as you remain consistent in the day you pick for your following experiments. To collect the mDCs, gently wash the culture dishes with several streams of media by pipetting up and down. The mDCs, which are currently immature, will be somewhat floating and only loosely adherent. Because of their loose adherence, they require several rounds of gentle pipetting, but do not require cell scraping, EDTA, or trypsin treatment. Note that the culture dishes will still contain some adherent cells. Do not worry about these cells, since these are not the loosely adherent DCs we are interested in.
Once the culture period has finished, between 6-8 days, the mDCs can be collected. The exact day is not critical, as long as you remain consistent in the day you pick for your following experiments. To collect the mDCs, gently wash the culture dishes with several streams of media by pipetting up and down. The mDCs, which are currently immature, will be somewhat floating and only loosely adherent. Because of their loose adherence, they require several rounds of gentle pipetting, but do not require cell scraping, EDTA, or trypsin treatment. Note that the culture dishes will still contain some adherent cells. Do not worry about these cells, since these are not the loosely adherent DCs we are interested in. Colt Egelston is currently a post-doctoral fellow at the Beckman Research Institute of the City of Hope, in Duarte, CA. He received his Ph.D. from Rush University in Chicago and is interested in all things immunology.
Colt Egelston is currently a post-doctoral fellow at the Beckman Research Institute of the City of Hope, in Duarte, CA. He received his Ph.D. from Rush University in Chicago and is interested in all things immunology.
