There are currently two immune cell targeting  immunotherapeutic agents that have received FDA approval for treatment of various malignancies. The first approved in 2010 was the autologous dendritic cell vaccine Provenge (Sipuleucel-T) by Dendreon Corporation, for hormone refractory metastatic prostate cancer. A second immunotherapeutic gaining FDA approval in 2011 for late stage melanoma, was an antibody called Ipilimumab, which inhibits CTLA-4, a major negative regulator of T cell activation. Antagonists to PD-1 such as Nivolumab and/or PD-L1, another receptor/ligand pair of T cell negative regulators, are expected to join this crowd by 2015. However, early results from combinatorial immunotherapeutics trials have demonstrated that significant synergy may be achieved by combining several immunotherapeutic modalities. A report in the June edition of The New England Journal of Medicine by Wolchok et al., demonstrates impressive synergistic results with combination therapy of Ipilimumab and Nivolumab in achieving deep and durable tumor regression in patients with advanced metastatic melanoma.
immunotherapeutic agents that have received FDA approval for treatment of various malignancies. The first approved in 2010 was the autologous dendritic cell vaccine Provenge (Sipuleucel-T) by Dendreon Corporation, for hormone refractory metastatic prostate cancer. A second immunotherapeutic gaining FDA approval in 2011 for late stage melanoma, was an antibody called Ipilimumab, which inhibits CTLA-4, a major negative regulator of T cell activation. Antagonists to PD-1 such as Nivolumab and/or PD-L1, another receptor/ligand pair of T cell negative regulators, are expected to join this crowd by 2015. However, early results from combinatorial immunotherapeutics trials have demonstrated that significant synergy may be achieved by combining several immunotherapeutic modalities. A report in the June edition of The New England Journal of Medicine by Wolchok et al., demonstrates impressive synergistic results with combination therapy of Ipilimumab and Nivolumab in achieving deep and durable tumor regression in patients with advanced metastatic melanoma.
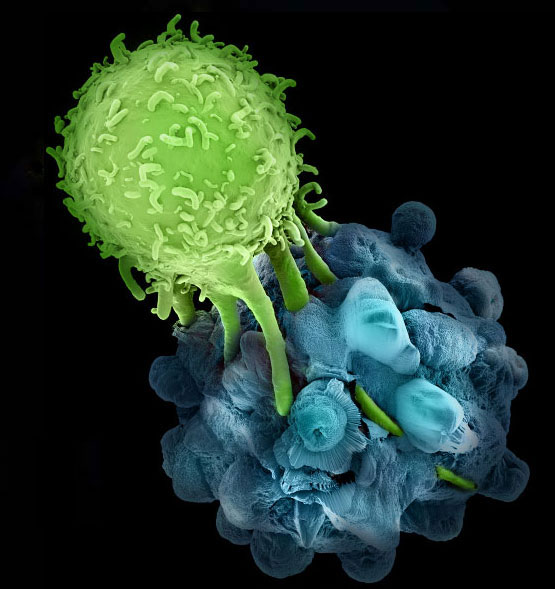
CTLA-4 and PD-1 are negative regulatory immune checkpoint inhibitors expressed on activated T cells and share homology with the TCR co-stimulatory receptor CD28. CTLA-4 strongly competes with CD28 for binding to CD80 (B7-1) and CD86 (B7-2) on antigen presenting cells thereby limiting CD28-activation signals, and furthermore recruits inhibitory molecules into the TCR signaling synapse. PD-1 interacts with ligands homologous with the B7 family, PD-L1 (B7H1) and PD-L2 (B7-DC), which are upregulated on tumor and stromal cells and activated antigen presenting cells. PD-1 interaction with its ligands leads to recruitment of SHP1 and SHP2 phosphatases to the immune synapse, resulting in inhibition of TCR-mediated signaling. PD-1 and CTLA-4 are considered non-redundant in their functions, and thus blocking both of these has been proposed to have a synergistic effect on T cell function in cancer, which was previously demonstrated in murine tumor models.
In previous clinical trials, Bristol-Myers Squibb’s Ipilimumab (MDX-010, Yervoy, IgG1) with or without a gp100 peptide vaccine extended overall survival of previously treated metastatic melanoma patients by almost 4 months versus gp100 peptide alone. In a second study, Ipilimumab plus darcarbazine versus darcarbazine alone was tested in previously untreated metastatic melanoma patients. The addition of Ipilimumab to the darcarbazine regimen extended overall survival by approximately two months, and lent to significantly higher survival rates at 1, 2, and 3 years later. Bristol-Myers Squibb’s PD-1 blocking antibody (BMS-936558, IgG4) showed significant clinical efficiency in metastatic or advanced non–small-cell lung cancer, melanoma, and renal-cell cancer.
In this dose-escalation phase I trial reported on by Wolchok et al., 53 patients with advanced melanoma were concurrently treated with Ipilimumab and Nivolumab, and 33 patients received sequenced treatment. Although the primary goal of a phase I trial is to evaluate safety, the clinical responses of the concurrently treated patients were exciting enough to garner much attention. In the concurrent regimen, 40% of patients had an objective response (modified WHO criteria), and 16 patients had a reduction in their tumor burden by 80% or more at 12 weeks, 5 being complete responses. Not only were responses faster and more pronounced than the previous clinical trials evaluating these inhibitors alone, but in the responding patients, the reduction in tumor burden was quite durable over the course of the study. Thus, results from phase III clinical trials comparing the combination to the inhibitors alone will be eagerly awaited. As an interesting note, tumor expression of PD-L1 has been proposed to be an indication of efficacy for Nivolumab. However, even in patients with PD-L1-negative tumors, responses to this combination regimen were observed.
Despite the strong promise of these inhibitors in combination, adverse events were notably higher than the inhibitors had exhibited alone in previous trials. Although no treatment-related deaths were reported, 72% of patients exhibited grade 3 or 4 adverse events, 53% of patients exhibited treatment-related grade 3 or 4 adverse events, and 21% of patients discontinued therapy due to treatment-related adverse events. Adverse events were however manageable with either immunosuppressant or hormone-replacement therapies.
Many different cancer immunotherapeutics are now being tested in clinical trials, including a number of therapies combining immunotherapeutic modalities in the hopes to achieve synergy. The results from the current trial indicate that anti-tumor T cells are not only present in tumor-bearing patients, but when uninhibited, can lend significantly to tumor-killing. This is truly an exciting time for cancer immunology.
Further Reading:
Nivolumab plus Ipilimumab in Advanced Melanoma. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. N Engl J Med. 2013 Jun 2.
Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. Topalian, S.L. et al. N. Engl. J. Med. 366, 2443–2454 (2012).
Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. Hodi, F.S. et al. N. Engl. J. Med. 363, 711–723 (2010).
Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. N Engl J Med. 2011 Jun 30;364(26):2517-26.
Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Lipson EJ, Drake CG. Clin Cancer Res. 2011 Nov 15;17(22):6958-62.