Antibodies play a significant role in countering foreign threats and ensuring specificity of self/non-self recognition. However, in healthy individuals, a substantial proportion of circulating IgM antibodies, secreted constitutively by B1 cells has been observed to demonstrate self-reactivity in the absence of antigen activation. These antibodies, which are present from birth without external stimulation, are known as natural auto-antibodies (NAA). Interestingly, such antibodies were found in human cord blood as well as in mice raised in germ-free conditions. One important feature of these molecules is their broad spectrum of reactivity to antigens ranging from proteins, polysaccharides and nucleotides to phospholipids. Though there are numerous reports that describe the crucial functions of NAAs in protection against infection, here I would like to discuss more about the role of these polymeric IgMs in maintaining tissue homeostasis and autoimmunity, with a brief look at the future of using this class of antibodies for therapeutic purposes.
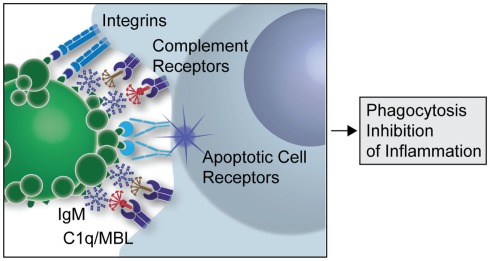
Though the immune system has strict mechanisms of ensuring removal of self reactive B cells to prevent autoimmune diseases, growing evidence suggests that auto IgMs produced by B1 cells play a major role in clearance of apoptotic cells (AC) and maintaining immune homeostasis. The innate immune system recognizes markers on cells destined to undergo apoptosis and clears them by a process called efferocytosis. Any defect in this clearance mechanism could lead to release and accumulation of autoantigenic proteins from these dying cells, resulting in the development of autoimmune diseases in predisposed individuals. A large number of the NAAs bind to oxidation associated markers like phosphorylcholine (PC) and malondialdehyde (MDA) that become exposed on apoptotic cells, but are conformationally inaccessible in the healthy ones. Recognition of such cells by auto IgMs promotes their complement mediated clearance (Fig. 1, Silverman 2012).
 Figure 1: A simplified overview of the clearance of apoptotic cells by natural IgMs via recruitment of complement factors (Silverman 2012)
Figure 1: A simplified overview of the clearance of apoptotic cells by natural IgMs via recruitment of complement factors (Silverman 2012)
Recent reports have demonstrated that mice engineered to be deficient in IgMs are more susceptible to autoimmune diseases resulting from the defective clearance of ACs. In other murine studies, it has been observed that IgMs against PC and MDA may lead to direct suppression of pro-inflammatory responses. In certain murine models of arthritis, infusion of anti PC natural IgMs conferred protection against inflammation. Also, in healthy humans a major portion of the natural IgMs is found to be generated against oxidation-associated epitopes on apoptotic cells. Indeed, recent studies in patients with systemic lupus erythromatosus substantiate the fact that higher levels of natural IgMs are associated with protection against autoimmunity. Moreover, several recent reports indicate that low levels of natural IgMs are associated with higher chances of atherosclerotic plaques, increased occurrence of strokes and even Alzheimer’s disease.
With growing evidence of the beneficial role of natural IgMs in autoimmunity and other diseases, efforts are on to develop effective therapeutic strategies based on the properties of these unique antibodies. In recent reports, different strategies such as injection of anti-idiotypic antibodies, administration of IL-18 and immunization with PC containing antigens have been described to increase natural IgM response. Moreover, pooled human IgM from healthy donors have been used with favorable effects in experimental autoimmune disease models. In fact, a preparation of IgG containing pooled 12% IgM is currently used for treatment of sepsis. In the future, further understanding of the role of these natural antibodies and their progenitor B cells could lead the way for the development of effective IgM based therapies against autoimmunity.
 Arijit Bhowmick is currently a postdoctoral researcher at the Immunology institute of the Mount Sinai Medical Center, NY. He received his PhD in structural immunology from the National Institute of Immunology, New Delhi. His current research interests encompass autoimmunity, Th17 cells and structure based inhibitor designing.
Arijit Bhowmick is currently a postdoctoral researcher at the Immunology institute of the Mount Sinai Medical Center, NY. He received his PhD in structural immunology from the National Institute of Immunology, New Delhi. His current research interests encompass autoimmunity, Th17 cells and structure based inhibitor designing.