Mesenchymal stem cells (MSCs) are multipotent cells present in, and can be isolated from a variety of adult tissues, such as bone marrow, umbilical cord blood or adipose tissue. MSCs have a number of advantageous characteristics that have made them a promising candidate for use in the new generation of cell-replacement therapy (CRT), even for central nervous system (CNS) disorders. Major obstacles to CRT in CNS disorders include successful delivery of therapeutic/stem cells to the damaged area/lesions within the CNS, host’s immune response against the allogenic cells and the possibility of ectopic tissue formation. MSCs exhibit unique characteristics which can overcome these obstacles, such as MSCs’ capacity to differentiate into multiple tissue-specific lineage cells, their role as progenitor-cell bioreactors of soluble factors that promote tissue regeneration from the damaged tissue and their immunomodulation capacity. Moreover, MSCs are considered immunoprivileged, meaning they have low expression of class II Major Histocompatibilty Complex (MHC-II) and other immune-stimulatory molecules on their cell surface. The focus of this discussion is on the utilization of MSCs in CNS-disease therapy, in particular multiple sclerosis (MS) and ischemic stroke (IS) .
Bone marrow derived (BM-MSCs) and adipose derived mesenchymal stem cells (AD-MSCs) have shown promising efficacy in an experimental autoimmune encephalomyelitis (EAE) preclinical model of MS, as well as the permanent middle cerebral artery occlusion (pMCAO) model of IS, respectively.
IS occurs as a result of an obstruction within a blood vessel supplying blood to a particular region of the brain, causing neuronal and astroglial damage within the immediate region. Replacement of these cells along with repairing the damaged tissue has been of great interest, turning IS clinical research towards stem cell therapy.
According to the recent study conducted by Gutierrez-Fernandez’s group, AD-MSCs are as restorative as BM-MSC in promoting recovery, repair and brain protection in IS rat models. They showed significant functional recovery, decreased apoptosis and increased expression of neurogenesis, synaptogenesis, angiogenesis and oligodendrogenesis markers, following the intravenous administration of allogenic AD-MSC and BM-MSC. Although these results suggest a less invasive route for administration of therapeutic cells, further studies addressing the fate of the administered cells are required, prior to clinical consideration of this method.
MS is a chronic, immune-mediated demyelinating disease of the CNS, characterized by demyelinated plaques within the brain and spinal cord. MS plaque formation consists of immune-cell infiltration, damage to oligodendrocytes and their subsequent failure to remyelinate, degeneration of axons, and ultimately astrocytosis. Up to date, there is no cure for MS; the current disease modifying therapies utilized to reduce the frequency/severity of relapses are limited to immunomodulatoion and are only partially effective. Thus, MS researchers have turned their attention to discovering potential therapeutics that will not only stop the autoimmune attacks, but also replace the destroyed CNS cells with properly functioning ones, through CRT.
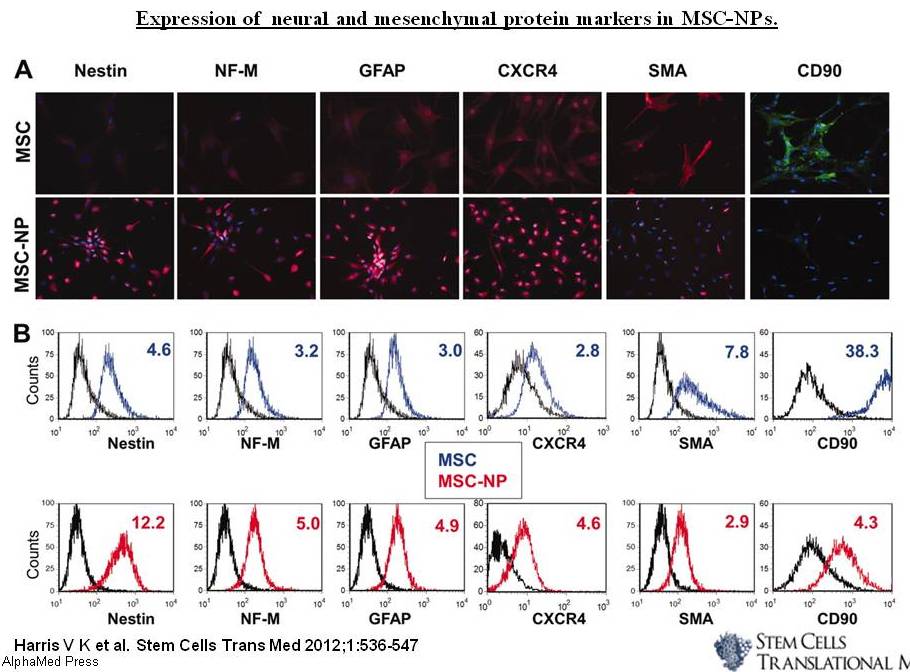
Nonetheless, several recent studies have reported promising results regarding autologous, culture-expanded MSC transplantation in MS models. Based on Harris’s publication in Stem Cells Translational Medicine, intrathecal delivery of Bone marrow mesenchymal stem cell-derived neural progenitors (MSC-NPs) is a promising strategy for cell-based therapy in MS. They show that MSC-NPs derived from both, MS patients and healthy controls, uniformly displayed properties that support MSCs’ therapeutic potential in the CNS, regardless of the donor disease status. Like MSCs, MSC-NPs secrete immunomodulatory factors (such as cytokines and growth factors, including TGF-β, IL-6, IL-10, HGF, heme oxygenase-1, and nitric oxide) which inhibited T-cell proliferation and promoted naïve CD4+ T-cell polarization into FoxP3+ T cells. MSC-NPs also exhibit trophic effects similar to MSCs, by secreting trophic factors that promote oligodendroglial differentiation of neural stem cells (HGF, IGF-1, SDF1α, and VEGF). Furthermore, it was reported that MSC-NPs are neuroectodermally committed and have reduced capacity for mesodermal differentiation (reduced risk of abnormal tissue formation), which makes them a more suitable candidate for cell-based therapy in CNS injury.
One of the significant aspects of this study is the possibility of utilizing MS-patient’s own cells as the therapeutic cell source; not only because of the reduced immune response, but also due to the evidence of genetic stability of the adult stem cells present in these patients. This suggests several promising genetic and cellular therapeutic strategies to be investigated in the near future.
Further readings:
Effects of intravenous administration of allogenic bone marrow- and adipose
Mesenchymal stem cell transplantation in multiple sclerosis.
Immunosuppressive properties of mesenchymal stem cells: advances and applications.


 536-47, Figure 3.jpg)