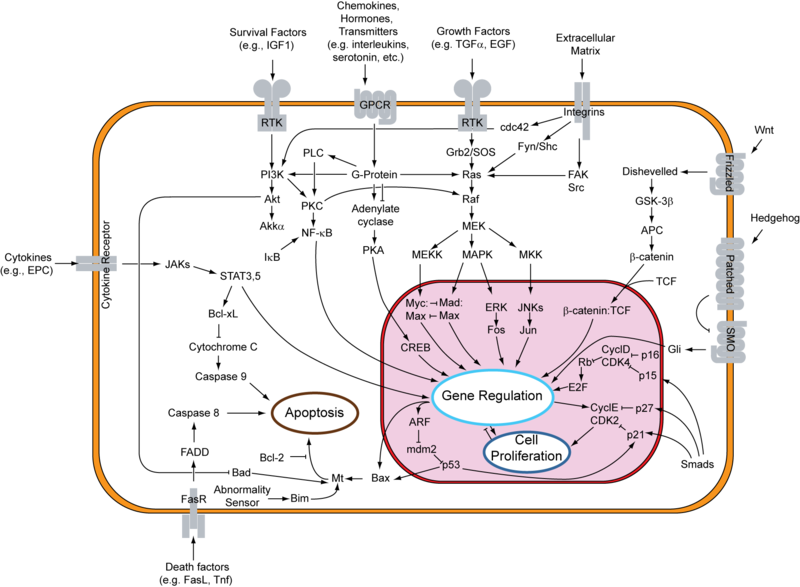
In recent years, “targeted” cancer therapies have shown promising results. With the discovery and development of “personalized” novel cancer treatments encouraging clinical responses in a subset of patients with advanced systemic disease have been observed.
This has been particularly evident with several of the recently developed kinase inhibitors that target oncogenic forms of EGFR, HER2, BCR-ABL, ALK, JAK2, and BRAF. In addition, several MEK inhibitors are developed to target oncogenic RAS. Although these drugs initially exhibit responses, they fail to sustain results due to the emergence of acquired resistance. The median duration of response with the EGFR inhibitor gefitinib (Iressa®, FDA approved) in non-small cell lung cancer patients is 7 months. Additionally, response duration for the BRAF inhibitor vemurafenib is approximately 6-7 months. In phase II clinical trials, median progression free survival time in patients bearing K-RAS mutant tumors treated with selumetinib (MEK inhibitor) was 5.3 months. Multiple mechanisms are associated to limiting the efficacy of these targeted agents. Therefore, further studies are needed to develop effective strategies to overcome the aquisition of resistance to targeted agents. Formulation of effective drug combinations as well as identification of novel compounds with anti-neoplastic properties could be useful in this context.
A recent study by Morris and colleagues published in Cancer Discovery (April 29th, 2013) reported the identification and characterization of a novel and selective ERK inhibitor. This inhibitor was found to induce tumor regression in BRAF, NRAS, and KRAS xenograft models and also inhibited MAPK signaling and cell proliferation in BRAF or MEK inhibitor resistant models. In addition, this ERK inhibitor was found to be effective in tumor cells that are resistant to concurrent treatment with BRAF and MEK inhibitors.
In this study, Morris et al. screened nearly 5 million compounds’ ability to bind the unphosphorylated form of ERK2 and observed ATP competitive compound, SCH772984, selectively bound and inhibited ERK1/2 activity at a nanomolar range. SCH772984 was effective in blocking ERK activation in a dose-dependent manner in V600EBRAF mutant and KRAS mutant cell lines. A known mechanism of resistance to MAPK inhibitors is via negative feedback activation of the MAPK pathway through ERK activation (for details please refer to my previous post titled “resistance to BRAF inhibitors in melanoma”). In this study, negative feedback activation via increased ERK phosphorylation was also observed following treatment with SCH772984. However, SCH772984 was found to be effective in blocking further downstream signaling of ERK as it maintained complete inhibition of ERK substrate p90 ribosomal S6 kinase.
Next, to test the efficacy of SCH772984 in the context of clinically observed resistance mechanisms to the MAPK inhibitors (such as BRAF amplification, MEK1 mutation, RAS mutation), researchers generated resistant cell lines and tested the efficacy of SCH772984. In all scenarios tested, sensitivity to SCH772984 treatment was observed as compared to the MAPK inhibitors. A recent clinical study concurrently treated patients with BRAF and MEK inhibitors and found to double the progression free survival period in patients whose tumors harbored MAPK activation especially in V600EBRAF mutant melanoma.
In the near future this combination could potentially become the standard-of-care for V600EBRAF mutant melanoma. However, due to heterogeneous nature of tumor cells, it is possible that acquired resistance may emerge to this combination treatment. To that end, Morris et al. tested the efficacy of SCH772984 in BRAF and MEK inhibitor combination resistant models of melanoma and colorectal cancer. Reduced activation of ERK and inhibition of cellular proliferation was noted in this model following SCH772984 treatment.
Taken together this study implicates a new ERK inhibitor SCH772984 that may exhibit encouraging therapeutic responses for the treatment of patients with BRAF, NRAS, and KRAS mutations including patients who relapse on current BRAF or MEK inhibitor therapy.
References:
1. Akinleye A, Furqan M, Mukhi N, Ravella P, Liu D. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27.
2. Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303-6.
3. Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013.