Natural killer (NK) cells represent up to 15% of human peripheral blood mononuclear cells (PBMC), and range from 5-20% of peripheral blood lymphocytes. NK cells generally fall into three subtypes: CD56dim CD16+, CD56brightCD16+/- and CD56– CD16+ NK cells, the prevalence and functions of which I have previously discussed. NK cells are considered to be a promising avenue in cell-based anti-tumor immunotherapeutics. However, the relatively low numbers of these cell types in PBMC have constituted a technical challenge in these efforts and in other studies needing large numbers of NK cells. In the April 2013 issue of Clinical & Experimental Immunology, Wang et. al, describe an in vitro method for the preferential expansion of human NK cells from PBMC.
NK cell expansion in vitro systems requires multiple signals for survival, proliferation, and activation. In a previous study, Fujisaki et. al (200 9) demonstrated that highly cytotoxic CD56+ NK cells could be highly preferentially expanded when cultured with a version of the chronic myeloid leukemia K562 cell line, which was genetically altered to express a membrane-bound form of IL-15 and the 41BB ligand (CD137L). Under this protocol, NK cells expanded an average of 21.6-fold after 7 days and 277-fold after 21 days in culture, and at 21 days reached a purity of 98.6%. CD3+ T cells on the other hand fell to an average of 3.1% of the cells remaining after 21 days. Importantly however, is not only the expansion of NK cells, but the functionality of the expanded cell product. The NK cells generated by this method had enhanced killing potential in vitro. In xenograft models of acute myeloid leukemia (AML) in immune deficient NOD/scid-IL2RGnull mice, these NK cells were able to elicit potent anti-leukemic activity. Thus, this method generates large numbers of highly functional human NK cells.
9) demonstrated that highly cytotoxic CD56+ NK cells could be highly preferentially expanded when cultured with a version of the chronic myeloid leukemia K562 cell line, which was genetically altered to express a membrane-bound form of IL-15 and the 41BB ligand (CD137L). Under this protocol, NK cells expanded an average of 21.6-fold after 7 days and 277-fold after 21 days in culture, and at 21 days reached a purity of 98.6%. CD3+ T cells on the other hand fell to an average of 3.1% of the cells remaining after 21 days. Importantly however, is not only the expansion of NK cells, but the functionality of the expanded cell product. The NK cells generated by this method had enhanced killing potential in vitro. In xenograft models of acute myeloid leukemia (AML) in immune deficient NOD/scid-IL2RGnull mice, these NK cells were able to elicit potent anti-leukemic activity. Thus, this method generates large numbers of highly functional human NK cells.
In the current study by Wang et. al, a similar method was utilized in which the K562 cell line was engineered to express a membrane-bound form of IL-21 along with CD137L. On average under these conditions, NK cells expanded from less than 30% of PBMC to over 85% after 7 days and 95% after 3 weeks, while CD3+ T cells went from 60% initially to 6% at seven days and 1% at three weeks. Proliferation of NK cells was continual over eight weeks in culture, and by 3 weeks reached over 100-fold, although the exact numbers and ranges were not explicitly stated in the paper. Thus, NK cells are highly selectively expanded using this method, similarly to the method used by Fujisaki et. al.
In answer to the functionality of NK cells generated under these conditions, Wang et. al demonstrated enhanced expression of activating and inhibitory NK receptors. Significantly enhanced cytotoxic killing potential after culture was shown, being maximal after one and three weeks in culture whereafter it decreased but still remained higher than resting NK cells. Thus, these expanded NK cells are also highly functional.
It would be interesting to see a direct comparison of the extent and quality of NK cell expansion from human PBMC by CD137L combined with the membrane-bound form of IL-15 as was done by Fujisaki et. al versus the membrane-bound form of IL-21 developed by Wang et. al. IL-21 is a strong and preferential activator of STAT3. Wang et al did establish a role for STAT3 in the induction of these cells. IL-15 is a strong activator of STAT5 and activates STAT3 to a lesser extent. However, IL-15 has been shown to strongly induce expression of the STAT3-activating cytokine IL-10. Thus, for optimal clinical applications of expanded NK cells, it is important to determine how the different cytokine-STAT signals contribute to NK cell proliferation, survival, and activation.
Further Reading:
Membrane-bound interleukin-21 and CD137 ligand induce functional human natural killer cells from peripheral blood mononuclear cells through STAT-3 activation. Wang X, Lee DA, Wang Y, Wang L, Yao Y, Lin Z, Cheng J, Zhu S. Clin Exp Immunol. 2013 Apr;172(1):104-12. doi: 10.1111/cei.12034.
Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Cancer Res. 2009 May 1;69(9):4010-7. doi: 10.1158/0008-5472.CAN-08-3712. Epub 2009 Apr 21.
Natural Killer Cell subtypes and markers in human PBMC
Types of immune cells present in human PBMC
Prospects for the use of NK cells in immunotherapy of human cancer. Ljunggren HG, Malmberg KJ. Nat Rev Immunol. 2007 May;7(5):329-39.
Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, Vánky F. Int J Cancer. 1976 Oct 15;18(4):421-31.
Characterization of cytokine differential induction of STAT complexes in primary human T and NK cells. Yu CR, Young HA, Ortaldo JR. J Leukoc Biol. 1998 Aug;64(2):245-58.
IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD, Choi I. Mol Cells. 2011 Sep;32(3):265-72. doi: 10.1007/s10059-011-1057-8. Epub 2011 Jul 29.

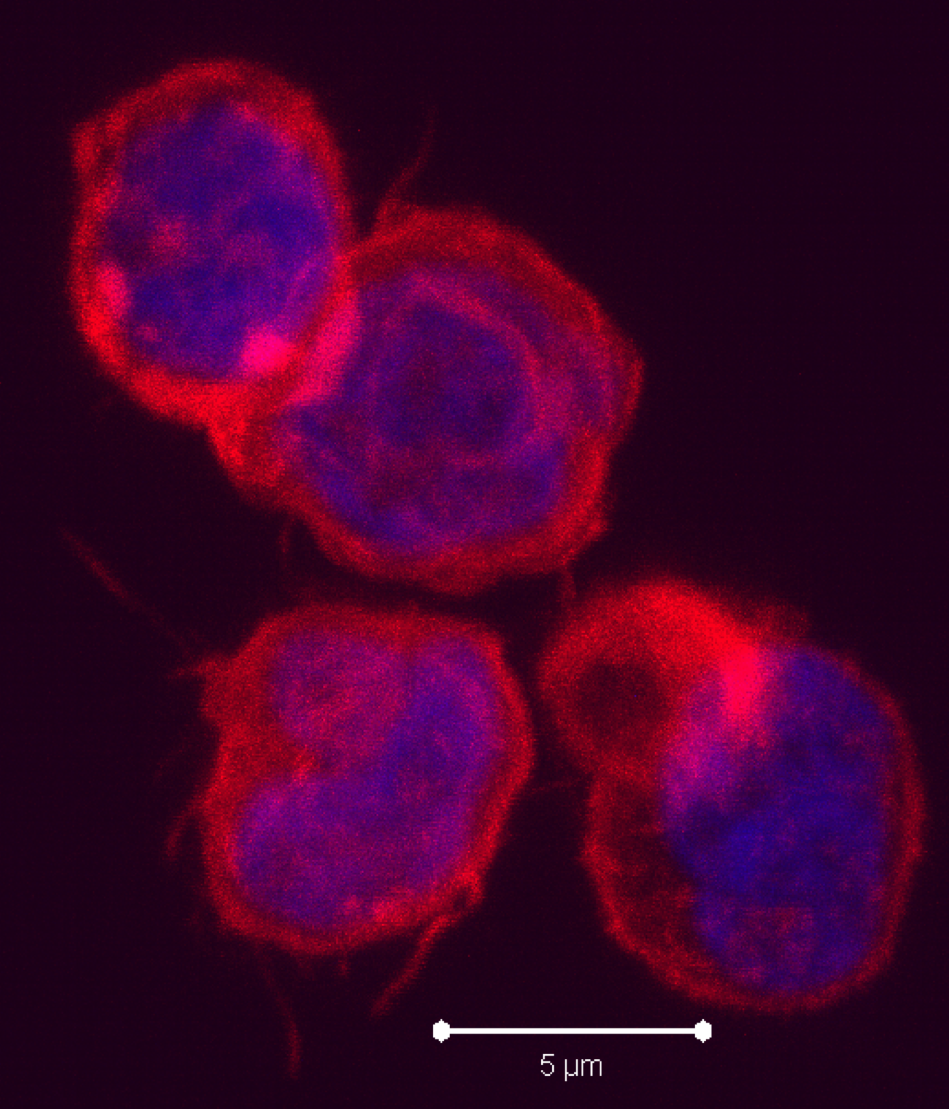

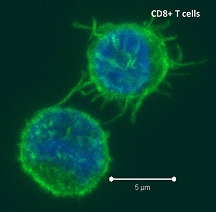
 Negative regulatory CD4 T cells are well characterized and highly studied. However their CD8 counterparts are not well defined, particularly in humans. Regulatory CD8 T cells suppress activated CD4 T cells and have proposed roles in various human diseases including multiple sclerosis, ovarian carcinoma and infection with HIV, and many subsets have been described using various markers. In a recent issue of PLoS One, Hu et. al, describe a population of CD3+CD8+CD161−CD56+ T cells within human peripheral blood mononuclear cells (PBMC) that exhibit a cytolytic negative regulatory function.
Negative regulatory CD4 T cells are well characterized and highly studied. However their CD8 counterparts are not well defined, particularly in humans. Regulatory CD8 T cells suppress activated CD4 T cells and have proposed roles in various human diseases including multiple sclerosis, ovarian carcinoma and infection with HIV, and many subsets have been described using various markers. In a recent issue of PLoS One, Hu et. al, describe a population of CD3+CD8+CD161−CD56+ T cells within human peripheral blood mononuclear cells (PBMC) that exhibit a cytolytic negative regulatory function.
 Flow cytometry has been around since the 1950s when Wallace Coulter developed the first flow cytometry device and fluorescence-based flow cytometry was introduced in 1968 by Wolfgang Göhde. Since then, fluorescence-based flow cytometry and fluorescence-activated cell sorting (FACS) have blown up to become a mainstay of analytical scientific approaches in every field of cell biology, especially immunology. However, the dominance of fluorescence-based flow cytometry for analytical cellular biology may change with the recent introduction of a new technology: Time of Flight Mass Cytometry (CyTOF).
Flow cytometry has been around since the 1950s when Wallace Coulter developed the first flow cytometry device and fluorescence-based flow cytometry was introduced in 1968 by Wolfgang Göhde. Since then, fluorescence-based flow cytometry and fluorescence-activated cell sorting (FACS) have blown up to become a mainstay of analytical scientific approaches in every field of cell biology, especially immunology. However, the dominance of fluorescence-based flow cytometry for analytical cellular biology may change with the recent introduction of a new technology: Time of Flight Mass Cytometry (CyTOF).

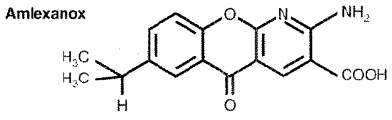
 Can amlexanox, a small molecule drug that has been approved for asthma, allergic rhinitis and aphthous ulcers be an effective treatment for obesity? Many are excited about this prospective presented in a recent article in Nature Medicine by Reilly et. al. as simply Googling “amlexanox” and “obesity” resulted in a plethora of news articles on this report. Interestingly, the two highly related proteins allegedly inhibited by amlexanox that led to this result in mice are the IRF3-kinases, TBK1 (TANK-binding Kinase-1, T2K, NAK) and IKK-ε (IκB kinase-epsilon, IKK-i), whose major known functions are during pathogen infections: the activation of IRF-3 (interferon regulatory kinase-3), the major transcription factor regulating expression of interferon-β (IFNβ).
Can amlexanox, a small molecule drug that has been approved for asthma, allergic rhinitis and aphthous ulcers be an effective treatment for obesity? Many are excited about this prospective presented in a recent article in Nature Medicine by Reilly et. al. as simply Googling “amlexanox” and “obesity” resulted in a plethora of news articles on this report. Interestingly, the two highly related proteins allegedly inhibited by amlexanox that led to this result in mice are the IRF3-kinases, TBK1 (TANK-binding Kinase-1, T2K, NAK) and IKK-ε (IκB kinase-epsilon, IKK-i), whose major known functions are during pathogen infections: the activation of IRF-3 (interferon regulatory kinase-3), the major transcription factor regulating expression of interferon-β (IFNβ).
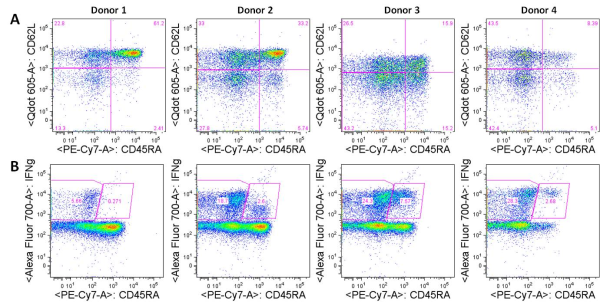
 CXCR5 is a chemokine receptor expressed by and used to identity human CD4+ follicular helper T cells (TFH). TFH cells, as their name implies, promote the differentiation and survival of memory and plasma B cells in the B cell follicular and germinal center regions of secondary lymphoid organs. CXCR5+ TFH-like central memory CD4+ T cells (CD4+ TCM) also circulate in peripheral blood and can be detected among human peripheral blood mononuclear cells (PBMC). CXCR5+ cells comprise 20-25% of CD4+ TCM cells in human PBMC. However, what are the identifying markers and functional differences of CXCR5+ vs. CXCR5– CD4+ T cells from human PBMC and the prototypical CXCR5+ TFH cells in secondary lymphoid organs?
CXCR5 is a chemokine receptor expressed by and used to identity human CD4+ follicular helper T cells (TFH). TFH cells, as their name implies, promote the differentiation and survival of memory and plasma B cells in the B cell follicular and germinal center regions of secondary lymphoid organs. CXCR5+ TFH-like central memory CD4+ T cells (CD4+ TCM) also circulate in peripheral blood and can be detected among human peripheral blood mononuclear cells (PBMC). CXCR5+ cells comprise 20-25% of CD4+ TCM cells in human PBMC. However, what are the identifying markers and functional differences of CXCR5+ vs. CXCR5– CD4+ T cells from human PBMC and the prototypical CXCR5+ TFH cells in secondary lymphoid organs?
 Natural Killer (NK) cells are a cytotoxic innate immune lymphocyte cell type. In humans, NK cells comprise up to 15% of peripheral blood mononuclear cells (PBMC), and 5-20% of the PBMC lymphocyte population. Several subtypes of NK cells exist in humans. In this post, I will discuss phenotypic properties and markers of NK subtypes present in human PBMC.
Natural Killer (NK) cells are a cytotoxic innate immune lymphocyte cell type. In humans, NK cells comprise up to 15% of peripheral blood mononuclear cells (PBMC), and 5-20% of the PBMC lymphocyte population. Several subtypes of NK cells exist in humans. In this post, I will discuss phenotypic properties and markers of NK subtypes present in human PBMC.
 Classical prognosis of cancer patients utilizes the AJCC/UICC (American Joint Committee on Cancer / International Union Against Cancer) “TNM” classification system, in which T (Tumor) is indicative of primary tumor size and invasion properties, N (Nodes) indicates the extent of tumor invasion into draining and regional lymph nodes, and M (Metastasis), describes the presence and extent of metastatic lesions at diagnosis. The combinations of these parameters are then used to assess a patient’s stage at diagnosis and predict patient outcome. The exact parameter definitions vary for each cancer type.
Classical prognosis of cancer patients utilizes the AJCC/UICC (American Joint Committee on Cancer / International Union Against Cancer) “TNM” classification system, in which T (Tumor) is indicative of primary tumor size and invasion properties, N (Nodes) indicates the extent of tumor invasion into draining and regional lymph nodes, and M (Metastasis), describes the presence and extent of metastatic lesions at diagnosis. The combinations of these parameters are then used to assess a patient’s stage at diagnosis and predict patient outcome. The exact parameter definitions vary for each cancer type.
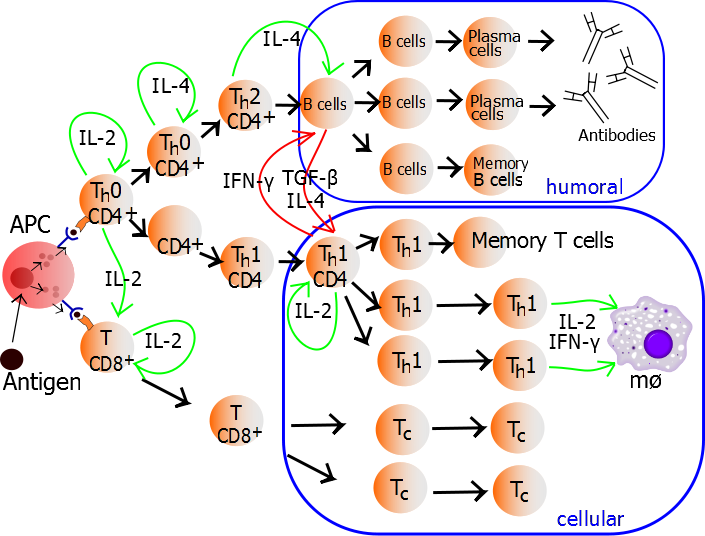
 TH1 cells can be generated and assayed for functions including IFNγ expression in as few as three days. If long term or clonal T cells assays are of interest, cells can be expanded in the presence of IL-2 for 2-3 weeks following single cell cloning.
TH1 cells can be generated and assayed for functions including IFNγ expression in as few as three days. If long term or clonal T cells assays are of interest, cells can be expanded in the presence of IL-2 for 2-3 weeks following single cell cloning.