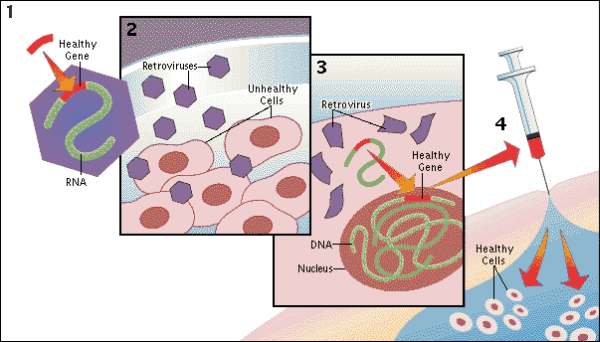
HSC gene therapy is an emerging therapeutic option for several disorders of the blood and immune system. Ex vivo cell therapies are based on the ability to isolate CD34+ cells from a patient or a normal donor, expansion ex vivo with genetic modification, and systemic administration into the patient following myeloablative treatment. An efficient method for gene transfer into HSCs is required for successful gene therapy. Lentiviral vectors (LVs) have emerged as a robust and versatile tool for ex vivo and in vivo gene delivery into multiple cell types, including HSCs. LVs can either be pseudotyped with viral envelope glycoproteins that confer a broad tropism, such as the vesicular stomatitis virus G (VSV-G) protein, or those that confer a specific HSC tropism, including gibbon ape leukemia virus (GALVTR), feline endogenous retrovirus RD114 (RD114TR), or amphotropic murine leukemia virus (MLV-A) proteins. However, viral envelopes vary in transduction efficiency. Thus, transduction protocols often involve the addition of factors to enhance viral entry, including cationic polymers (polybrene) 1 or fibronectin fragments (Retronectin) 2.
Recently, in Molecular Therapy-Nucleic Acids, Fenard et al identified another viral entry enhancer, Vectofusin-1 3. Vectofusin-1 is a synthetic, histidine-rich cationic amphipathic peptide derived from the LAH4 peptide family. LAH4 peptides and their derivatives are known to be efficient DNA transfection agents 4. In this study, the authors examined whether Vectofusin-1 would also enhance gene transfer of LVs into CD34+ cells derived from human umbilical cord blood. Indeed, Vectofusin-1 significantly increased the transduction efficiency of LVs pseudotyped with various envelopes (VSV-G, GALVTR, RD114TR, MLV), with transduction levels ranging from 50-80% compared to undetectable transduction levels in its absence. In addition, the increased transduction efficiency was not cytotoxic. Addition of Vectofusin-1 during transduction of CD34+ cells did not negatively affect subsequent myeloerythroid differentiation in colony-forming cell (CFC) assays in vitro, or hematopoietic reconstitution in immunodeficient BALB-Rag/γC mice in vivo. The mechanism for the increased transduction efficiency was attributed to insertion of the peptide in the viral and cellular membranes, resulting in an enhancement in both adhesion and fusion of the viral particles with the cell’s plasma membrane.
In short, the authors demonstrated that Vectofusin-1 is a promising LV entry enhancer that can be potentially used in ex vivo transduction of HSCs for subsequent use in clinical applications. Addition of Vectofusin-1 to the transduction medium had similar effects as the commonly used Retronectin, although the latter is used to coat plates, suggesting a different mechanism of action. Future experiments will determine whether Vectofusin-1 and Retronectin can be used together to synergistically enhance HSC transduction.
References:
1 Davis, H. E., Morgan, J. R. & Yarmush, M. L. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophys Chem 97, 159-172 (2002).
2 Pollok, K. E. & Williams, D. A. Facilitation of retrovirus-mediated gene transfer into hematopoietic stem and progenitor cells and peripheral blood T-lymphocytes utilizing recombinant fibronectin fragments. Curr Opin Mol Ther 1, 595-604 (1999).
3 Fenard, D. et al. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol Ther Nucleic Acids 2, e90, doi:10.1038/mtna.2013.17 (2013).
4 Kichler, A., Leborgne, C., Marz, J., Danos, O. & Bechinger, B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci U S A 100, 1564-1568, doi:10.1073/pnas.0337677100 (2003).