Thyroid cancer is a form of tumor growth located within the thyroid gland. It is the most prevalent endocrine malignancy and affects both men and women at any age. During the early stages of the disease, many patients do not experience symptoms. However, as the cancer progresses, symptoms can include a lump or nodule in the front of the neck, hoarseness or difficulty speaking, swollen lymph nodes, difficulty swallowing or breathing, and pain in the throat or neck. There are different types of thyroid cancer: papillary, follicular, medullary, anaplastic, and variants. Among these, differentiated thyroid carcinoma, namely papillary and follicular thyroid carcinoma, makes up about 94% of these cases.
Radioactive iodine (RAI) treatment is given to treat differentiated thyroid carcinoma. The treatment uses a radioactive form of iodine called iodine 131 or I-131. The RAI circulates throughout the body in the bloodstream. Thyroid cancer cells pick up the RAI wherever they are present in the body. The radiation in the iodine then kills the cancer cells. This is a targeted treatment. It doesn’t affect other body cells, because only thyroid cells take up the RAI. Even though differentiated thyroid cancer is curable, recurrence occurs in 20-40% patients. In about 5% patients with cellular dedifferentiation, the disease develops more aggressive behavior and metastatic growth. This results in tumor resistance to conventional therapy, RAI uptake, and poor prognosis. Resistance to the RAI therapy has been reported to be responsible for a large number of deaths in advanced thyroid carcinomas (papillary and follicular), and represents a major clinical challenge.
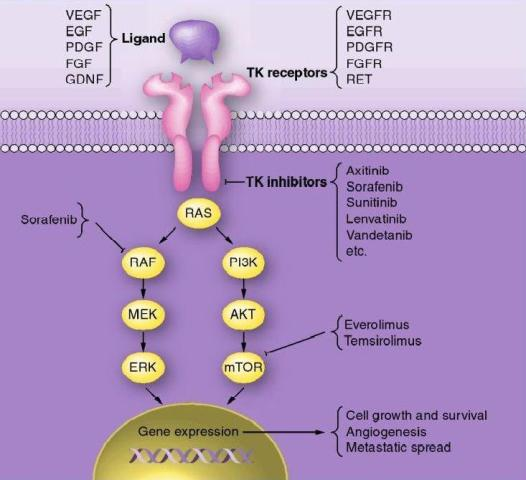
Approximately 70% papillary thyroid carcinoma is typically associated with mutations in RET,  RAS and BRAF oncogenes. In follicular thyroid carcinoma mutations of RAS oncogene in addition to other gene mutations was also observed. Aberrant activities of these oncogenes results in constitutive activation of the MAPK-pathway leading to inhibition of sodium-iodide symporter and thyroid peroxidase genes which participate in iodide uptake and thyroid hormone production respectively. In a pre-clinical study, Chakravarty et al. (2011) showed that mice with poorly differentiating thyroid cancer and overexpressing BRAF (V600E) oncogene failed to uptake RAI. Shutting BRAF activation off or inhibiting the MAPK-pathway with kinase inhibitors targeting BRAF or MEK rendered mice susceptible to a therapeutic dose of RAI. This suggests that inhibition of the MAPK-pathway and its associated protein kinases with the protein kinase inhibitors may facilitate RAI uptake in refractory thyroid cancer patients harboring MAPK-pathway activation.
RAS and BRAF oncogenes. In follicular thyroid carcinoma mutations of RAS oncogene in addition to other gene mutations was also observed. Aberrant activities of these oncogenes results in constitutive activation of the MAPK-pathway leading to inhibition of sodium-iodide symporter and thyroid peroxidase genes which participate in iodide uptake and thyroid hormone production respectively. In a pre-clinical study, Chakravarty et al. (2011) showed that mice with poorly differentiating thyroid cancer and overexpressing BRAF (V600E) oncogene failed to uptake RAI. Shutting BRAF activation off or inhibiting the MAPK-pathway with kinase inhibitors targeting BRAF or MEK rendered mice susceptible to a therapeutic dose of RAI. This suggests that inhibition of the MAPK-pathway and its associated protein kinases with the protein kinase inhibitors may facilitate RAI uptake in refractory thyroid cancer patients harboring MAPK-pathway activation.
A pilot clinical study published recently in The New England Journal of Medicine (368;7 February 14, 2013) by Ho et al. observed that inhibition of the MAPK pathway with MEK inhibitor selumetinib (AZD6244) enhanced RAI uptake in thyroid cancer patients that are 
In addition to the activation of oncogenes, overexpressions of many tyrosine kinase receptors (c-Met, EGF, VEGF etc) has also been reported in dedifferentiated thyroid cancers. The signaling cascades of these receptors cause constitutive activation of both MAPK and PI3K/Akt pathway and thereby disrupting iodine transport and thyroid hormonogenesis. This further suggests that pharmacological abrogation of the MAPK pathway and also PI3K/Akt pathway may be clinically beneficial in RAI-refractory thyroid cancer. Several clinical trials are currently evaluating efficacy of various protein kinase inhibitors (GSK2118436, Sorafenib, Everolimus, Lenvatinib) targeting these pathways to re-sensitize RAI-refractory thyroid carcinomas to radioactive iodine therapy.
Suggested reading:
1. Chakravarty, D., Santos, E., Ryder, M., Knauf, J. A., Liao, X. H., West, B. L., Bollag, G., Kolesnick, R., Thin, T. H., Rosen, N., et al. (2011). Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest 121, 4700-4711.
2. Ho, A. L., Grewal, R. K., Leboeuf, R., Sherman, E. J., Pfister, D. G., Deandreis, D., Pentlow, K. S., Zanzonico, P. B., Haque, S., Gavane, S., et al. (2013). Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368, 623-632.
3. http://clinicaltrials.gov/ct2/results?term=refractory+thyroid+cancer&pg=1